Unboxing the next frontier in transplant medicine
Signals Brief: A novel transplant endpoint; how it works; and why it might change the course of decision-making and drug development in kidney transplantation
This edition of Signals was made free for all readers thanks to Roivios, the team developing the world’s first renal assist device. Check out JuxtaFlow, a clinical-stage solution for renal insufficiency. Thanks team!
In February 2021, after months of pandemic restrictions, Leila Mirhaydari was finally able to see her kidney doctor in person again—a huge relief after being unable to get the in-person care she needed for so long. But with that relief came anxiety. Transplanted organs require careful monitoring, and Leila had been looking after her transplanted kidney on her own for a year. So much was riding on those first lab results.
"Immediately, all my levels were just out of whack and I knew that I was in rejection," she says. "I've had to work through a lot of emotional pain, of feeling like I failed my donor. Like, why couldn't I hold on to this kidney?"
Her step-cousin’s donated kidney had allowed Leila to be free of dialysis, to travel the world, and live her life for eight "beautiful years." Now it seemed that time was coming to an end. Leila began preparing to go through the arduous journey of waiting for another transplant.
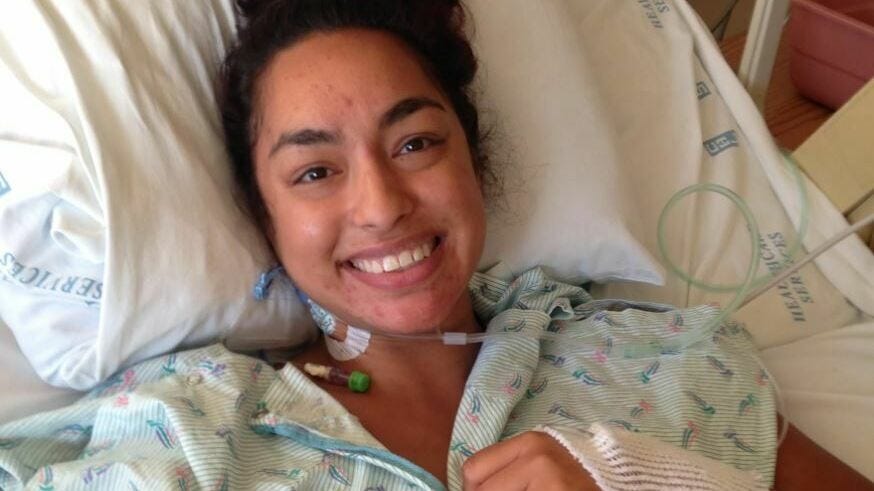
Leila’s experience highlights the reality for many kidney transplant recipients: while kidney transplants offer life-saving benefits, they are not a permanent cure. On average, a living donor kidney lasts 15 to 20 years, while a deceased donor kidney lasts 8 to 12 years. Some will last longer; others less. The uncertainty of kidney survival underscores the need for better tools to predict long-term outcomes after surgery— and it gets at the heart of what we’re talking about today.1
While Leila was facing kidney rejection, a global team of researchers over 7,000 miles away set out to better understand how we might predict the long-term success of kidneys after transplant— what’s known as graft survival. Their work has laid the foundation for a new metric that might help us cut through the fog in the distance once and for all.
The iBox Scoring System was developed to address a major gap in kidney transplantation: the lack of reliable early surrogate endpoints to support drug discovery, clinical trials, and long-term graft survival.2
Predicting long-term success with short-term data alone has proven difficult and unreliable. We are limited by our current toolset and time constraints. This is why tools like iBox hold so much potential—not only in reshaping transplant medicine, but also in helping us realize a long-held vision of giving every recipient “one kidney for life.”3
Today, let’s begin to answer three important questions:
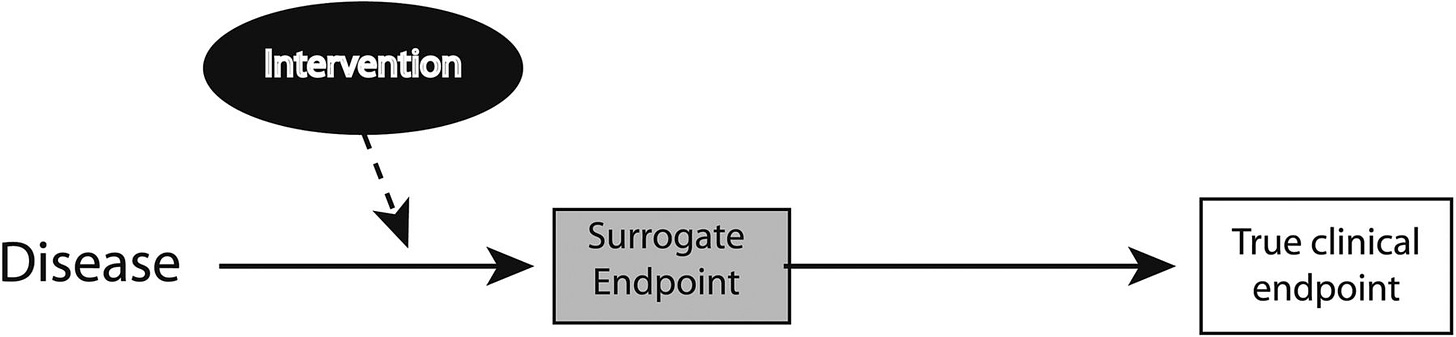
What are surrogate endpoints, and why do we need them?
How would iBox improve decision-making in transplant care?
Why would iBox unlock new drug development and innovation?
What are surrogate endpoints, and why do we need them?
Surrogate endpoints are lab measures or physical signs used as substitutes for direct clinical outcomes. While they don’t measure the actual clinical benefit of a therapy, they are designed to predict it.
Why do we need them? Well, clinical outcomes may take years to observe, which could delay a therapy being available to patients who would benefit. As you can imagine, this becomes important in areas like kidney transplant, where much of our focus (and the system itself) is centered on short-term outcomes (i.e. 1-yr, 5-yr), while longer term outcomes can be little better than a coin flip in some cases. The 5 and 10 years graft survival rates are 77% and 49% for deceased donor and 86% and 64% for living donor transplants. This stark contrast makes it difficult to develop and test new drugs or therapies that could help us improve graft survival over longer time horizons— say 8, 10, or ever 20+ years beyond surgery.4
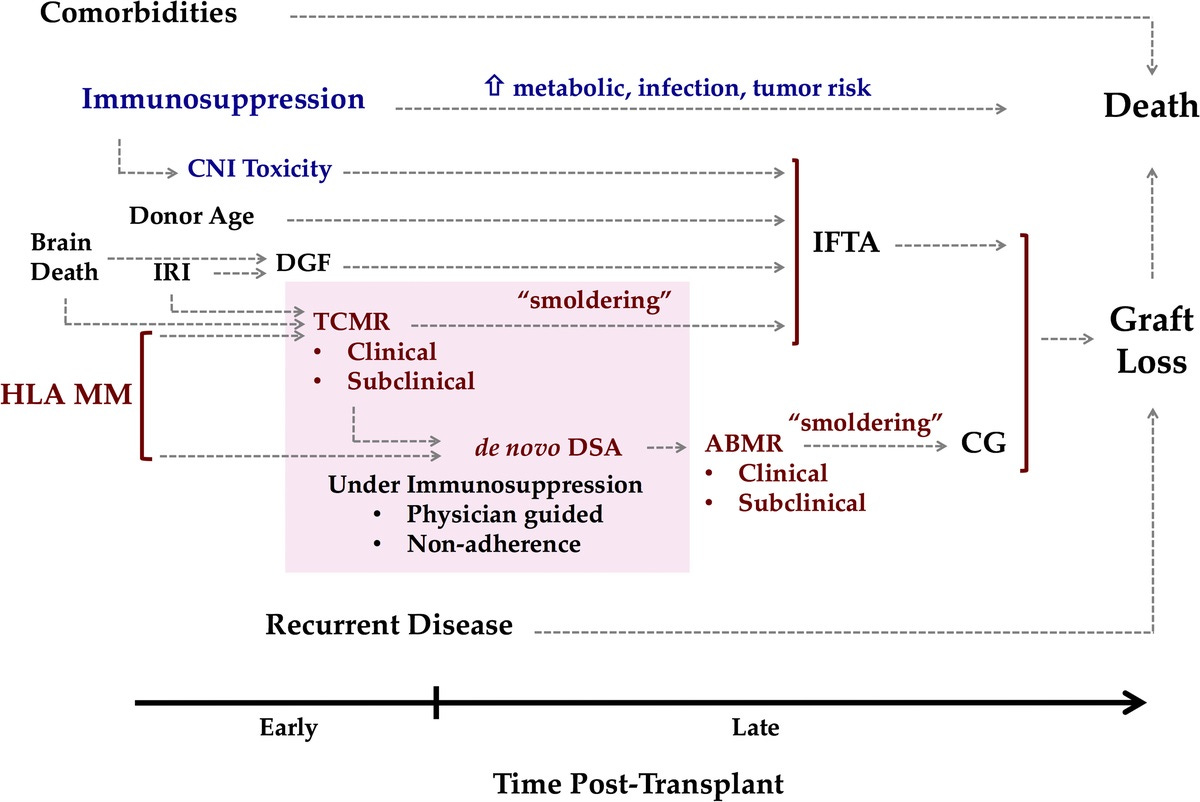
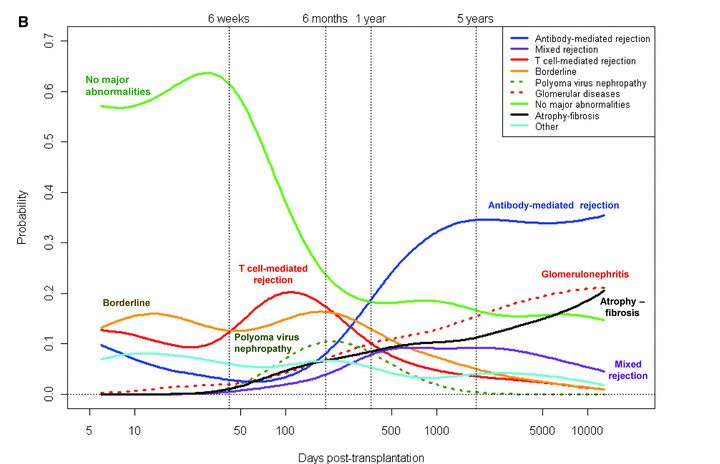
This complexity arises because late graft failure is often multifactorial—a result of several interconnected factors, such as immune response, rejection, and side effects of medications, making prediction difficult. In short: graft loss is complicated. A 2016 paper in Transplantation captures this well (see figure below), showing pathways to graft loss related to immunosuppression (blue) and those linked to graft immune response (red). We’ll cover some of these factors and how they play into decision-making in the next section. Simply put, short-term surrogate endpoints don’t capture the range of variables that influence long-term graft survival. This is why we need a more comprehensive model to predict long-term success.5
In clinical trials, surrogate endpoints can help catalyze the approval process. The FDA’s Accelerated Approval Program uses surrogate endpoints to approve drugs for serious conditions faster, allowing researchers to get treatments to patients sooner while still ensuring they’re safe and effective. For instance, reducing systolic blood pressure can predict stroke risk, allowing trials to be completed more quickly with smaller populations.67
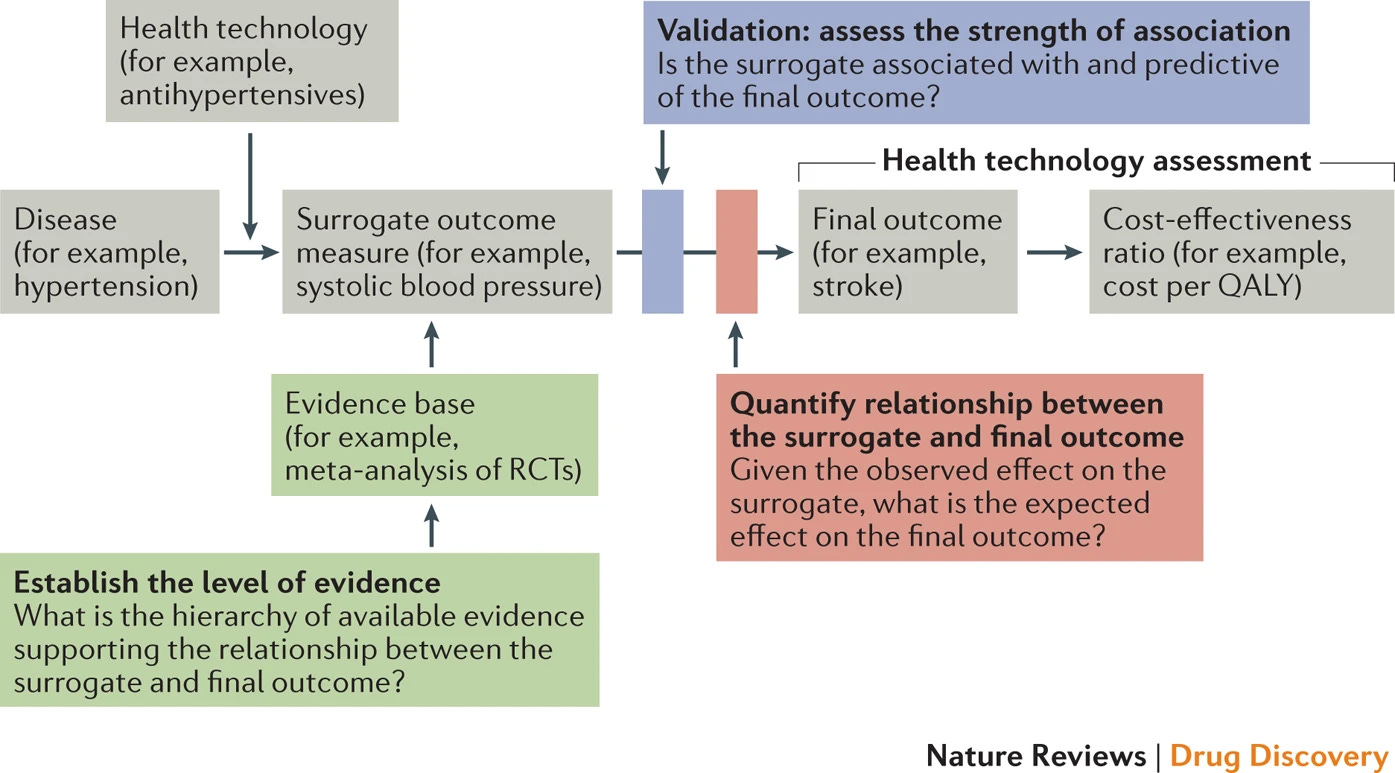
However, surrogate endpoints must be carefully validated to truly predict the desired clinical outcome. In kidney transplantation, the lack of reliable long-term predictors has been a major challenge. Traditional endpoints fail to reliably predict long-term graft survival across organ types, leaving a gap for patients like Leila and Amy, who are at risk of graft failure.
“I’ve had the opportunity to sit in on a few closed-door meetings of professional transplant organizations where physicians speak about the problem openly, if briefly, in a safe space for voicing regret and frustration. They admit with shrugged shoulders that after the first five years post-transplant, they don’t know how much immunosuppressive medicine will keep a transplanted organ protected and a recipient’s body safe from harm.” — Amy Silverstein (April, 2023)

This is where the iBox Scoring System aims to fill a vast unknown. Developed by Dr. Alex Loupy and the team at PARCC— and with support from C-Path’s Transplant Therapeutics Consortium— the iBox score combines eGFR, proteinuria, histology, and HLA-DSA to predict long-term graft failure. This model offers a more comprehensive view of risk and outperforms traditional methods. By integrating multiple risk factors, the iBox model provides better insights into a kidney’s long-term survival, which is crucial for improving patient care, drug development, and clinical trial design.89
In 2022, iBox was endorsed and qualified by the European Medicines Agency (EMA), and just last month, it received FDA acceptance of its Biomarker Qualification Plan. This marks a significant milestone in the progression of the iBox system, as it continues to gain recognition on both sides of the Atlantic as a key tool in kidney transplant clinical trials. The Paris Transplant Group is the multidisciplinary team behind iBox. They bring together immunologists, researchers, nephrologists, cardiologists, pathologists, statisticians, and public health specialists to advance the field of transplantation. They also put together this helpful 2-minute video overview of the iBox algorithm for those interested.
How can iBox improve decision-making in transplant care?
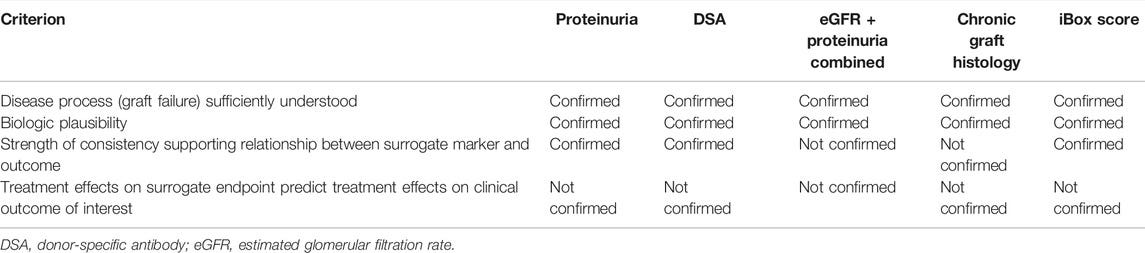
The Transplant Therapeutics Consortium (TTC) aims to quality iBox as a surrogate endpoint for long-term graft survival. In transplant medicine, decisions are often based on short-term outcomes, such as 1-year graft survival, which are important yet incomplete reflections of the full picture. As we’ve covered, the challenge with predicting long-term outcomes lies in the complexity of late graft failure, influenced by a host of factors from immune response and medication side effects to underlying health conditions. However, it’s important to note that individual measures—such as eGFR (estimated glomerular filtration rate) and proteinuria—are insufficient on their own to capture the full range of long-term risks, making it challenging to predict graft survival over time based on any single measure (see below table).1011
A second clear issue is the inconsistency in how different physicians interpret the same clinical data. A recent study comparing iBox to physician decision-making highlighted this problem. Physicians showed wide variability in their predictions, depending on which risk factors they prioritized (e.g., IFTA vs. ABMR), leading to inconsistent (and sometimes inaccurate) assessments. Dr. Alex Loupy, one of the study authors, points out that this variability is a clear indication of weak calibration and intra- and inter-observer variability. Unlike humans, who interpret data inconsistently, iBox provides a stable, data-driven prediction model that helps clinicians make more informed and consistent decisions. In the figure below, researchers asked 9 physicians to rank the 10 most important features driving their predictions across 400 patients. The features (y-axis) are presented from descending importance. The higher the value of mean decrease accuracy (x-axis), the higher the importance of the feature in the model. Take an extra second to ponder this one.
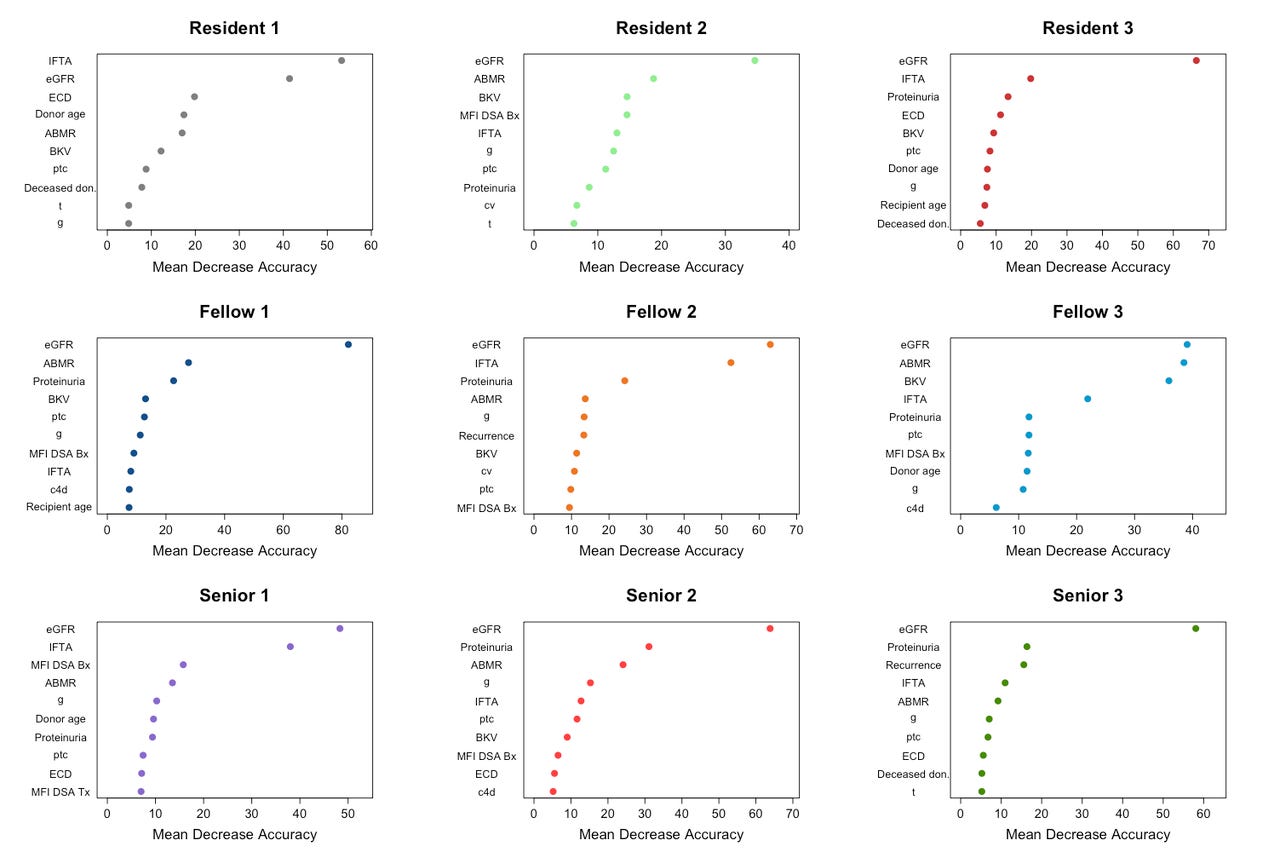
The iBox score stands out by combining several key factors known to affect long-term kidney function. It takes into account eGFR, proteinuria, histology (biopsy results), and donor-specific antibodies (DSAs). By combining these factors, iBox gives a more complete picture of a transplant recipient’s risk, which allows clinicians to make more informed decisions about care. This comprehensive model accounts for more variables than traditional methods, which often rely solely on eGFR or proteinuria to assess graft function. iBox’s ability to incorporate a combination of clinical, histological, and immunological data sets it apart, offering a more robust, reliable prediction of long-term graft survival.
Loupy and colleagues compared the predicted probabilities of kidney survival generated by physicians versus the iBox system. Their results (below) show a much more consistent prediction distribution from iBox, compared to the varied predictions from different physicians. Again, this demonstrates iBox’s potential to improve decision-making by offering a stable and reliable prediction model, reducing the variability inherent in human assessments.
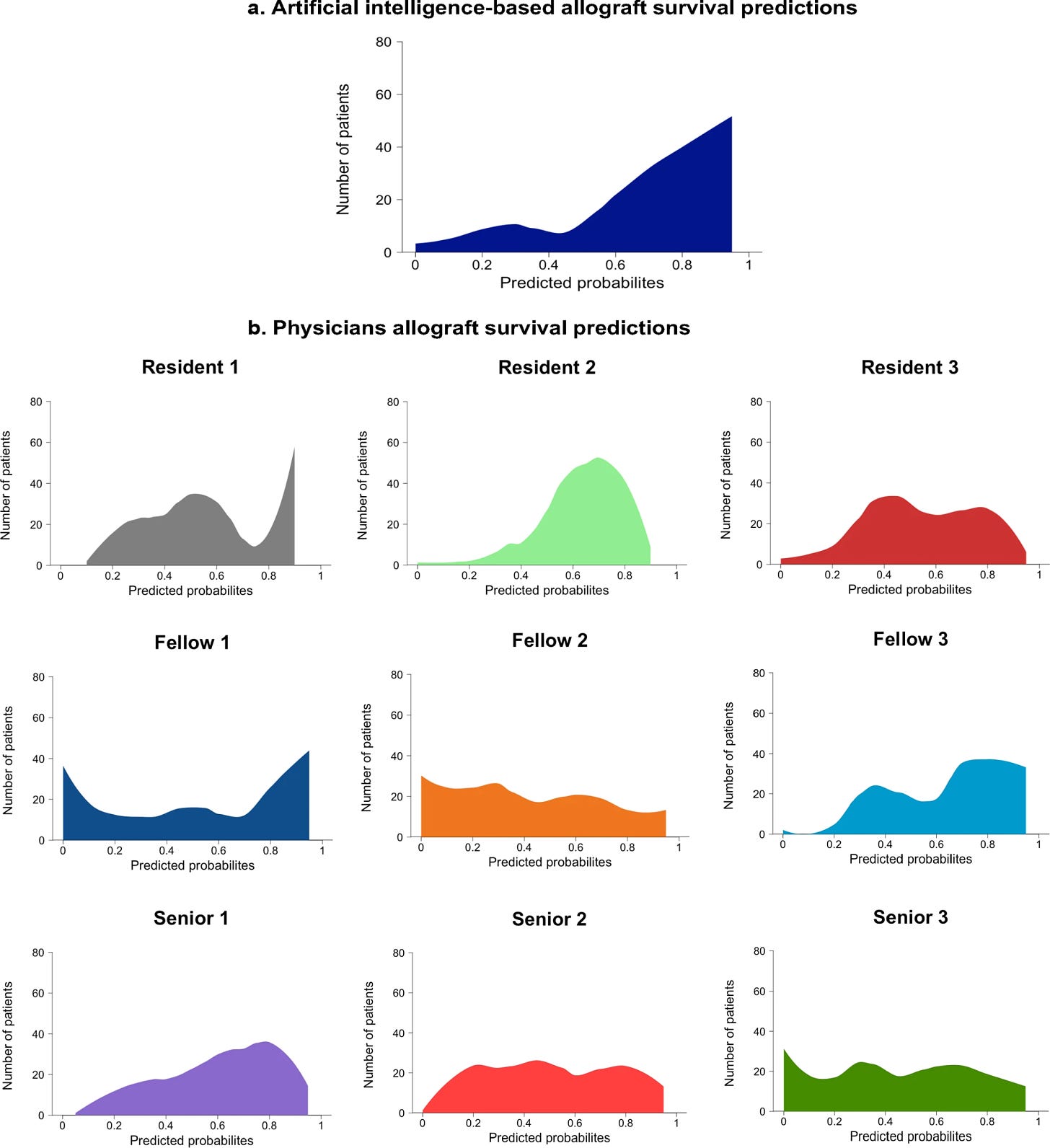
iBox also improves upon the status quo by providing a tool that not only assesses non-inferiority, as is common in clinical trials, but also demonstrates the superiority of new immunosuppressive therapies (ISTs). By offering a more reliable prediction of long-term graft survival, iBox is now positioned to serve as a key secondary endpoint in clinical trials, particularly those evaluating therapies aimed at improving long-term kidney transplant outcomes. Its qualification enables its use in pivotal or exploratory drug studies, assessing treatment effects from 6 months to 2 years post-transplant—a critical period for understanding long-term impacts on graft survival. This longer time frame provides a more meaningful measure of success compared to traditional 1-year endpoints.12
This is especially important because, historically, there has been little incentive for drug developers to focus on long-term outcomes beyond 5 years. Current endpoints typically demonstrate non-inferiority rather than superiority, and the lack of reliable long-term surrogate endpoints has hindered the development of drugs that might improve kidney survival over the long term. By providing a more reliable method for predicting long-term success, iBox could fill this gap, helping clinicians and drug developers alike focus on the bigger picture of improving graft survival for years beyond the immediate post-transplant period.
Recent studies show iBox’s ability to bridge the gap between short-term and long-term outcomes (here, here, and here). By improving the accuracy of risk prediction, it helps reduce physician variability in making decisions and offers a more objective approach to patient care.
iBox’s regulatory momentum further backs its importance in transplant medicine. In 2022, the European Medicines Agency (EMA) qualified iBox for use in clinical trials, and now, the FDA has accepted iBox as a surrogate endpoint for kidney transplant clinical trials. This recognition is a major milestone and paves the way for its use in accelerating the development of new therapies. By offering more reliable predictions and minimizing human variability, iBox could change the way clinical trials are designed and help speed up the development of new treatments. Ultimately, this shift could lead to better, more personalized patient care, and more consistent transplant outcomes.
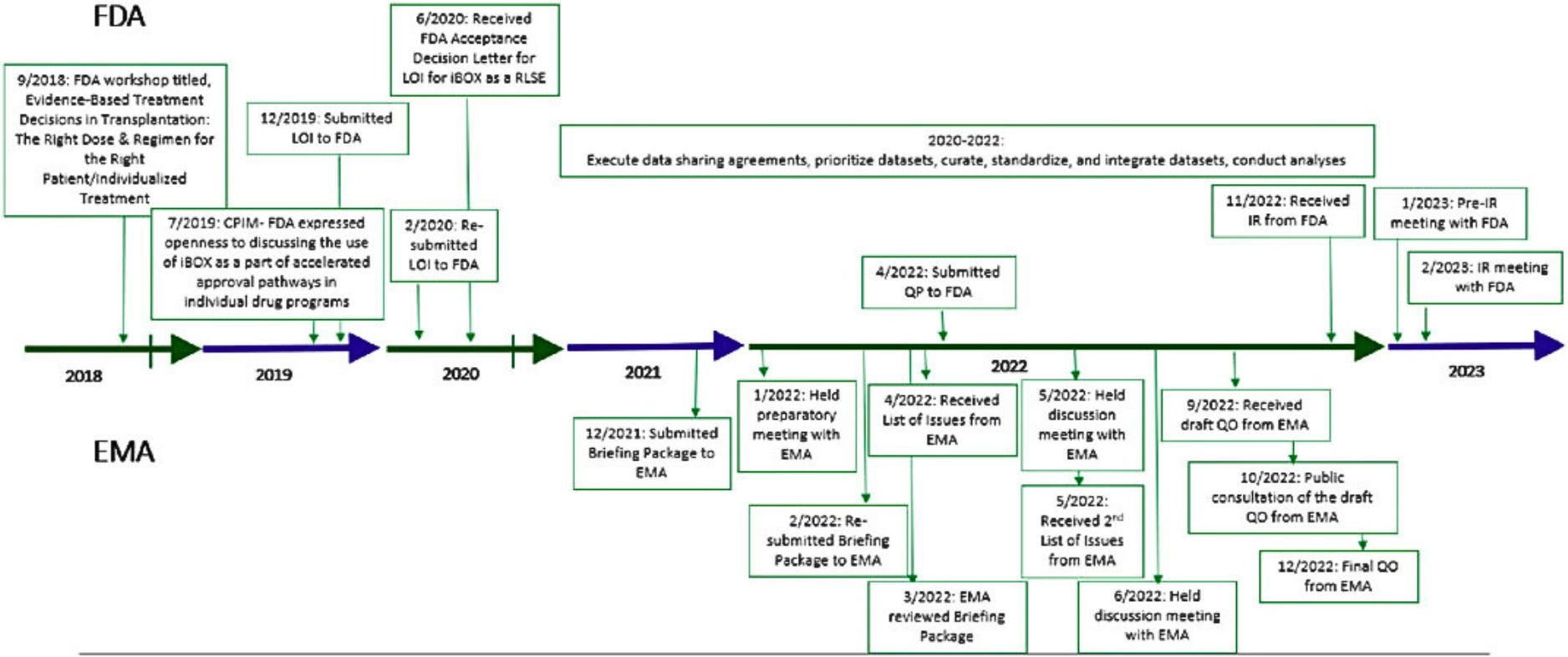
Kevin Fowler, a patient advocate and kidney transplant recipient, pointed out that while iBox has the potential to improve transplantation, it took the FDA 12 months to decide to review it. As the process continues, Fowler emphasizes that the kidney transplant community wants to be part of this review. For comparison, it took iBox one year from initial submission to receiving final QO from Europe’s regulatory body (EMA) in December, 2022 (see below).
What would this mean for drug discovery, clinical trials, and innovation?
The regulatory framework for kidney transplant therapies has long been focused on preventing organ rejection. However, no therapy has been approved for preventing long-term graft loss, which remains a critical gap in transplant medicine. Currently, all FDA-approved immunosuppressive therapies (ISTs) are indicated for the prophylaxis of organ rejection. This regulatory framework has centered around immediate post-transplant survival rather than the long-term health of the transplant, and, as a result, we have basically “maximized” 1-year graft survival as an endpoint for clinical trials (see below). Going a bit further, drug developers face few incentives to pursue treatments that aim to improve long-term graft survival.
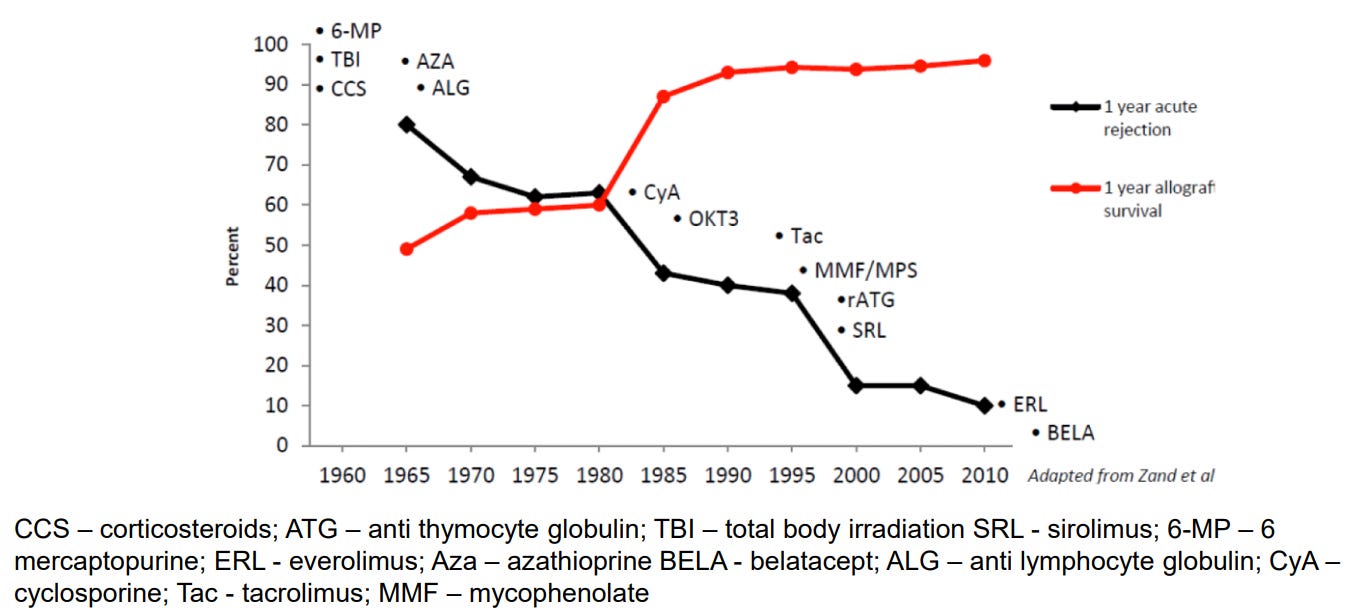
This regulatory limitation has stifled the development of new therapies for kidney transplants. Over the past two decades, no new IST demonstrating improved efficacy has been developed, and no new IST has been approved for the prevention of organ rejection for more than a decade. There are also no new ISTs in Phase 3 clinical trials today. With iBox now qualified as a surrogate endpoint for clinical trials, particularly for the critical 6-month to 2-year period post-transplant, there is new hope for accelerating the development of therapies that target long-term transplant outcomes. iBox offers a tool to bridge the gap between short-term efficacy and long-term graft survival, enabling the development of new therapies that could help extend the life of kidney transplants and improve long-term outcomes for recipients.13
What if a new drug could be indicated for the prophylaxis of organ rejection and improvement in the iBox in kidney transplant?
This is not yet a reality, but it’s an example of what iBox’s prognostic ability—compared to markers like BPAR (biopsy-proven acute rejection)— could mean for potentially qualifying iBox as a reasonable likely surrogate endpoint (RLSE). This could provide sponsors with a pathway for accelerated approval through the FDA.
There’s precedent for this, including right here in the Kidneyverse. Surrogate endpoints have played a critical role in accelerating the availability of new treatments in areas like oncology, HIV, Alzheimer’s, sickle cell disease, and Fabry disease. IgA nephropathy serves as a prime example of what can happen when sponsors have the right endpoints in place. In 2020, there were no approved treatments for IgA nephropathy. As of writing, three IgAN treatments have been approved—Tarpeyo (2021), Filspari (2023), and Fabhalta (2024)— with at least 10 more in development. For a disease that affects 130,000 to 150,000 people in the US, up to 40% of whom will develop kidney failure within 10 to 20 years of diagnosis, this is the kind of progress that serves as a guiding light for the future.14
What’s Next
The qualification of iBox marks a critical milestone, bringing us closer to therapies that could significantly extend the lives of kidney transplant recipients and help us realize the ‘one kidney for life’ dream. As the first transplant endpoint to reach this stage, iBox is now positioned to be used as a co-primary endpoint in pivotal clinical trials and the Accelerated Approval Pathway at the FDA. This pathway fast-tracks approval for drugs addressing serious conditions and unmet medical needs, offering hope for quicker access to life-changing treatments.
The next step is the submission of the Full Qualification Package to the FDA, which will further establish iBox as a reliable tool for predicting long-term graft survival and improving transplant care. With ongoing research involving thousands of transplant recipients, the team behind iBox will continue to evolve, adapt, and solidify its role in clinical practice and drug development. The Transplant Therapeutics Consortium (TTC) and its partners have been instrumental in achieving this progress, and their collaboration will accelerate the development of new therapies.
Discussion
This win for iBox has significant implications for transplant medicine, drug development, patient outcomes, and regulatory pathways. How do you think about these questions?
Surrogate Endpoints in Transplantation: How will surrogate endpoints like iBox transform the landscape of transplant medicine? Which other endpoints should we be aware of or paying attention to?
Accelerating Drug Development: How will iBox’s FDA qualification open the door to faster approval for new immunosuppressive therapies? When do you think we will begin to see the benefits of iBox as a novel endpoint?
Long-Term Patient Outcomes: What would iBox approval mean to you in terms of what it does in clinical practice or drug development? How could this change the way you approach patient care, trial design, or market assessment?
Impact on Regulatory Processes: How might iBox change the way we evaluate kidney transplant therapies? Do you expect to see any changes in regulatory pathways as a result of iBox? What should we keep an eye on as the next Administration takes office in 2025?
As always, thank you for being here with us. If this conversation resonates, or if you have a story or point of view to share, we’d love to hear from you in the comments below. And if you want to join your peers in the brand new Signals slack community, we’d love to have you— please join the waitlist here.
— Tim
Resources & Data
At the end of each brief I like to share a short list of sites, studies, reports, and other interesting gems I came across while researching the piece. Enjoy!
Are Long-Term Outcomes after Kidney Transplantation Improving? The FDA hosted a public workshop in November, 2023 to discuss endpoints and trial designs to advance drug development in kidney transplant. Part of that workshop included presentations by Drs. Emilio Poggio, Ergun Velidedeoglu, Jeff Siegel, and Paul Conway to name a few. This single doc (yes, it is really 327 slides) has perspectives from FDA, academia, patient advocates, developers and other key stakeholders in the endpoints field. It contains enough data, charts, and insights to get you up to speed on subtopics while also giving you plenty of new threads to pull on. Enjoy!
My Transplanted Heart and I Will Die Soon. This stirring, beautiful guest essay by Amy Silverstein was published in The New York Times last April. In it, she explains that “over the last almost four decades a toxic triad of immuno-suppressive medicines — calcineurin inhibitors, antimetabolites, steroids — has remained essentially the same with limited exceptions. These transplant drugs cause secondary diseases and dangerous conditions, including diabetes, uncontrollable high blood pressure, kidney damage and failure, serious infections and cancers. Transplantation is no different from lifelong illnesses that need newer, safer, more effective medicines. The key difference is that only in transplantation are patients expected to see their disease state as a “miracle…”
Farewell, my kidney: Why the body may reject a lifesaving organ. Leila’s story at the top of this piece was captured and shared by the NPR Short Wave team in May of 2023. It’s fantastic, and well worth a read or listen.
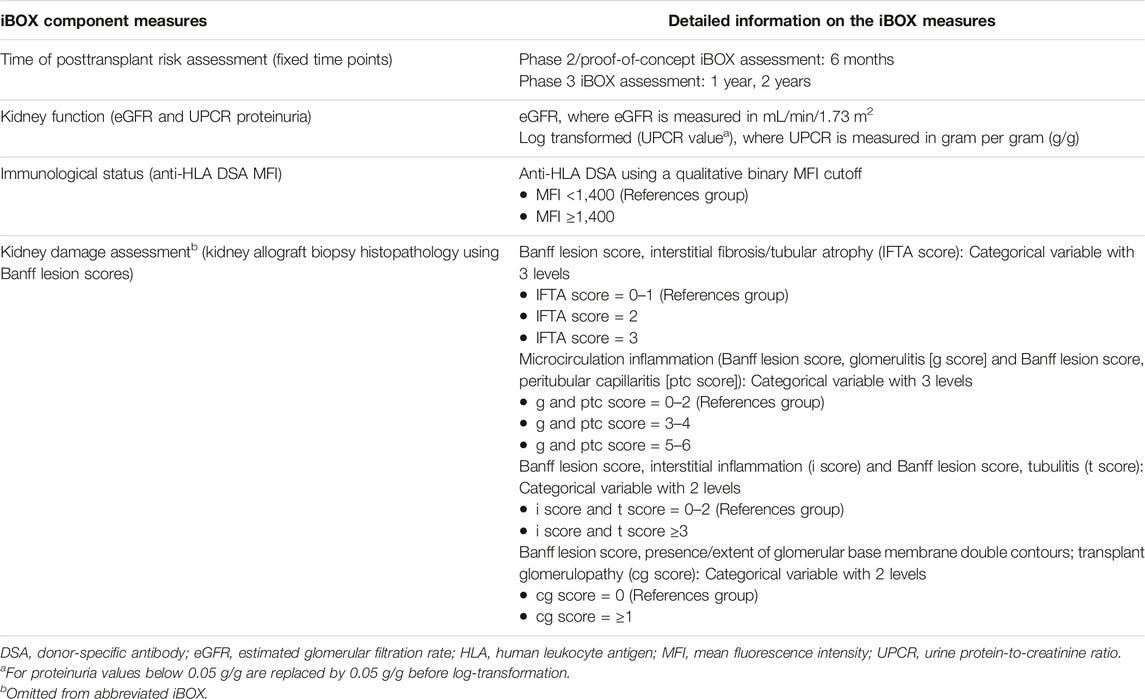
What goes into the iBOX algorithm anyways? Here are the component measures of the full and abbreviated iBOX.
Want to support Signals?
Farewell, my kidney: Why the body may reject a lifesaving organ — NPR (May, 2023)
One Kidney for Life. Montgomery, R.A. American Journal of Transplantation, Volume 14, Issue 7, 1473 - 1474
Naesens M, Budde K, Hilbrands L, Oberbauer R, Bellini MI, Glotz D, Grinyó J, Heemann U, Jochmans I, Pengel L, Reinders M, Schneeberger S and Loupy A (2022) Surrogate Endpoints for Late Kidney Transplantation Failure. Transpl Int 35:10136. doi: 10.3389/ti.2022.10136
Wiebe, Chris MD; Nickerson, Peter MD. Strategic Use of Epitope Matching to Improve Outcomes. Transplantation 100(10):p 2048-2052, October 2016. | DOI: 10.1097/TP.0000000000001284
Accelerated Approval Program — FDA.gov
The research team at Paris Translational Research Center for Organ Transplantation (PARCC) is developing a personalized approach of transplant medicine that will integrate multi-dimensional information deriving from classical histology and biology, clinical science together with novel information coming from ground breaking technologies in immunology, molecular biology, genetics and biomarkers. The iBox project has the ambitious but realistic goal to provide transplant clinicians with innovative and accessible tools for early prediction of individual risk of allograft rejection and transplant loss and offering them the possibility to personalize clinical management and treatment. Learn more here.
Critical Path Institute (C-Path) is supported by the FDA and is 54% funded by the FDA/HHS, and 46% funded by non-government source(s). C-Path’s Transplant Therapeutics Consortium (TTC) is the only community-based group dedicated to advancing the regulatory science needs of transplant. Learn more here.
Hartung EA. Biomarkers and surrogate endpoints in kidney disease. Pediatr Nephrol. 2016 Mar;31(3):381-91. doi: 10.1007/s00467-015-3104-8. Epub 2015 May 16. PMID: 25980469; PMCID: PMC4646734.
Naesens M., et al.
For more details on the differences between non-inferiority (NI) and superiority trials, and considerations in determining a non-inferiority margin, please refer to Slides 220-239 of the Endpoints and Trial Designs to Advance Drug Development in Kidney Transplantation from the November, 2023 workshop.
Ibid. (Slide 114)
![Signals From [Space]](https://substackcdn.com/image/fetch/w_80,h_80,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F55686857-6b99-45a6-ac0f-09c9f023f2a0_500x500.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/e_trim:10:white/e_trim:10:transparent/h_72,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F4d588ac1-7fac-4bd4-829d-fc7b4e8f1326_1512x288.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/w_36,h_36,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F55686857-6b99-45a6-ac0f-09c9f023f2a0_500x500.png)