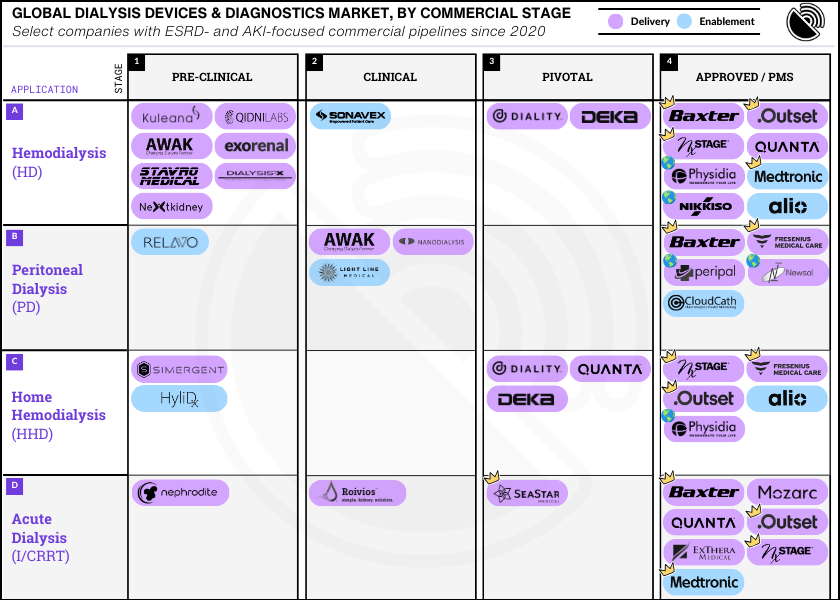
Expert Q&A: Bringing New Devices To Market In Kidney Failure (ESKD)
How 6 device leaders think about progress, pitfalls, and potential in this space
Welcome to the second edition of Signals Expert Q&A, the series that that asks industry leaders to weigh-in on pitfalls, progress, and potential in their respective areas of expertise in the Kidneyverse.
This month we’re bringing together some of the brightest minds and boldest innovations in the landscape of end stage kidney disease, or ESKD. Bringing a medical device to market in this space is a herculean feat that requires capital, grit, teamwork, and patience.
Today we’ll learn how six experts think about device innovation in end stage kidney disease. Over the course of seven questions you’ll get to know the problems they’re tackling, the solutions they’re working on, and how they think about devices in the broader context of policy, regulatory strategy, and commercialization.
Whether you are an industry veteran or just starting out, this Signals Q&A will give you an inside look at how device innovation gets done in kidney care.
Meet our experts
Our experts represent a broad range of insights and experiences. They have founded, led, and advised companies at every stage of the commercial lifecycle. Their diverse paths to the medical device space span backgrounds in nephrology, vascular access, clinical research, banking, and consulting. Today, they shape the visions and strategies of products that aim to improve patients’ lives across the globe. I’m grateful for their leadership and for sharing their earned wisdom with all of us today.
John E. Milad, Former Co-Founder & CEO at Quanta
Michael Serle, Clinical Director at Mozarc Medical
Dr. Cynthia Silva, VP Clinical Affairs at Outset
Alex Hassan, Head of Product at Mendaera
Eric Schlorff, CEO at SeaStar Medical
David Narrow, CEO at Sonavex
What you’ll learn
Q&A
Q1. At the highest level, how would you describe current approaches, devices, and treatment options in your space?
TLDR: Our experts describe a transformative phase in kidney care, marked by the advent of portable devices and innovative home therapy options. The field, which had seen minimal innovation for decades, is now experiencing a surge in technological advancements addressing dialysis access, infection control, remote patient monitoring, and improving the overall quality of life for CKD patients. Despite significant advancements, home adoption rates remain low due to educational gaps and patient apprehension. There's a consensus on the need for standardization and quantitative approaches over traditional qualitative ones to improve outcomes. The current focus spans across a spectrum from wearable artificial kidneys to devices that ensure safe cannulation, highlighting a commitment to not only enhancing treatment efficiency but also patient comfort and safety.
John: A new generation of portable hemodialysis devices is more effectively addressing the barriers to providing more patients with the benefits of home dialysis Importantly, these technology changes are being integrated within improved systems-based approaches to optimize patient journeys and care pathways.
Michael: Until very recently, kidney care had experienced a notable lack of significant innovation for several decades. However, in the past few years there has been a noticeable shift, with numerous medical device companies and startups taking on the challenge of driving innovation. Companies are tackling the challenges around dialysis access creation and maintenance, identifying infection control solutions, employing remote patient monitoring, and investigating home therapy delivery options. Given that chronic kidney disease (CKD) patients represent one of the most vulnerable patient populations, they deserve new technologies to optimize their disease management – improving clinical outcomes and enhancing their quality of life.
Cynthia: Home hemodialysis (HHD) was long dormant with little innovation, and the past few years have seen a promising turn with new ideas entering the space. Tablo was one of the first major innovations in decades, designed with the patient in mind, to improve the dialysis experience and reduce the cost of care for providers. Tablo is one of two devices currently approved for hemodialysis in the home and has the largest usability study published. Meaning there is data showing patients, caregivers and providers find this new device is safe, effective and easy to learn. Despite this data, less than 3% of Americans are on HHD due to lack of knowledge, exposure from their healthcare team or fear of needles.
Alex: Despite there being over 65 million cannulation attempts per year in the U.S., safe and effective fistula identification, assessment, and cannulation remains more art than science for ESRD patients and providers. There are recommendations and techniques for improving outcomes, but there is little standardization across providers — a challenge exacerbated by nearly continuous staffing turnover at many (if not most) ESRD care sites — and most of these techniques being qualitative heuristic-based approaches rather than quantitative ones. As such, we should not be surprised that access-related infection and injury remain problems for ESRD patients. Devices to improve these issues, including imaging devices and novel needle designs, have been tried, but many have fallen by the wayside as 'failed experiments' — not because they didn't work, but because they were hard to adopt into the current ESRD care paradigm.
Eric: Many of the current approaches in the ESRD space are focused on either creating artificial wearable kidneys, increasing portability and access through home hemodialysis, improving clearance, or on bothersome symptoms like pruritus that patients face.
David: There are a variety of new and emerging devices that cover the continuum of access creation, maintenance, assessment, surveillance and cannulation. Many of these hold great promise and the community is eagerly awaiting the evidence needed to bring these technologies to patients.
Q2. What do you see as the biggest barriers to safe and effective use of devices in ESRD settings today?
TLDR: The experts cite several significant barriers, including late diagnosis of kidney disease, care coordination issues, institutional biases, and insufficient home dialysis programs. Training and education around new devices present challenges due to technical complexities and high staff turnover. Economic hurdles such as misaligned reimbursement structures, cost-benefit analyses, and the need for proven economic value further complicate the integration of new technologies. Additionally, patient awareness, access to certified home dialysis facilities, fear of self-cannulation, and regulatory concerns are key obstacles that hinder adoption rates. These factors collectively contribute to a resistance to adopting innovative treatments, especially in underserved regions and among vulnerable patient populations.
John: The barriers to better ESRD treatment are multifactorial and start with the fact that many patients don't know they have advanced kidney disease until they crash land in the hospital with kidney failure. Other factors include poor care coordination and multidisciplinary care; institutional bias against home therapies, which are shown to deliver better clinical outcomes and to be cost effective; lack of critical mass in home programs; misaligned reimbursement structures and payment incentives; and, historically, incomplete device and service offerings from vendors.
Michael: Two of the biggest barriers when it comes to effective use of medical devices in ESRD settings, from my perspective, are (i) the availability of effective training and education, and (ii) cost and reimbursement.
The ability to effectively educate large numbers of dialysis care providers on new technology can be a challenge. There can be a steep technical learning curve for some devices, or a limited number of field specialists to offer in-depth training for other technologies. Both issues can translate to a delay in technology integration.
Cost and reimbursement challenges cannot be ignored. A significant amount of time, money and resources go into the development of medical devices including R&D, clinical testing, and regulatory approval to name a few. Once commercialized, widespread patient access to more expensive technologies frequently varies widely based on the site of service due to payer reimbursement which can ultimately impact treatment options for the patient. We often see this amplified particularly in underserved populations and in regions with limited healthcare resources. It is also worth noting for those readers that are unfamiliar with the capitated payment structure for ESRD care specifically in the US, integrating new technology often translates to increased treatment costs without additional reimbursement. This can certainly present a challenge for technological innovation.
Cynthia:
(i) Awareness – Most dialysis patients are unaware that home dialysis is even an option for them! That they can indeed be successful.
(ii) Access – only 54% of dialysis facilities are certified by Medicare to offer home dialysis and only 27.5% of facilities are certified to offer home hemodialysis. More rural areas have even less access to dialysis facilities. This coupled with facilities not offering home dialysis creates a bottleneck.12
(iii) Fear of cannulation – Needle phobia and burden on care givers are not well addressed in terms of novel approaches or creating support (dollars, support groups, free education).
(iv) Clinic Staffing challenges – Sending patients "Home" is a more personalized approach and entails a multidisciplinary team to help make the patients successful. Currently going in center is the "easy" button. Not all clinics are equipped to manage home dialysis patients or are only equipped to manage a certain number of home dialysis patients at any given time.
Alex: Significant barriers to broad adoption of new devices in ESRD care are (i) cost and cost-benefit analysis and (ii) device education and perceptions of workflow disruption.
(i) Cost-benefits — Low per-encounter margins make even relatively inexpensive devices unpalatable. For example, using real-time imaging to evaluate fistula maturity or using plastic needles for cannulation are proven techniques with price points that would be considered "very low" in other parts of the healthcare system, yet cost is often cited as a reason why they are not widely adopted in ESRD care. Difficulty in calculating the "benefit" in a cost-benefit analysis on new devices in ESRD can also limit adoption. It is often easier for manufacturers to prove clinical benefits than financial benefits when bringing products to market; until devices can be deployed in a large clinical population, financial benefits often remain unproven, which in turn makes it hard for providers to invest in them. Achieving the critical mass of patients in order to prove the benefit then becomes difficult or impossible — and the cycle continues.
(ii) Device education — Users need to be educated to use new devices safely and effectively. High staffing turnover amongst ESRD providers undermines training efforts by manufacturers and limits the ability for new technology to take hold at care sites. New devices are also perceived to cause disruptions to existing workflow, at least during the initial adoption phase. Clinical staff are often under high production pressure and having to slow down to learn and use a new technology can cause significant resistance to adoption. When users are caregivers or patients, there are additional challenges educating them on how to use medical devices at all— that is, if they comfortable trying to do so in the first place.
Eric: Biggest barriers to effective use of medical devices include technology limitations, stakeholder understanding of new technologies, regulatory concerns over long term uses of certain devices, and a general lack of new targets that can be intervened in.
David: The primary challenge relates to market access and reimbursement considerations. Many technologies demonstrate clinical benefit, achieve regulatory approvals, and garner the interest of key influencers and thought leaders. However, most fall short due to the lack of proven economic value and/or lack of separate payment.
Q3. What can be done today to overcome these barriers? Who will need to be involved? What does a great outcome look like?
TLDR: The group suggests a multi-faceted approach centered around education, innovation, and collaboration. Codifying best practices into a consistent operating system, engaging in cross-sector partnerships, and leveraging diverse information outlets are key strategies. Manufacturers, providers, and policymakers must collaborate, with manufacturers considering economic constraints in design and policymakers incentivizing innovation. Providers play a vital role in embracing new technologies and contributing to evidence generation. A great outcome is a healthcare environment where multidisciplinary collaboration leads to evidence-based strategies and market access, supported by payors and regulatory bodies.
John: We already know what best practice looks like from decades of experience around the world. We need to codify that learning into an operating system that consistently puts this into practice for every patient.
Michael: Kidney health technology companies like Mozarc Medical invest a great deal of time and resources into building expert training and education teams to develop effective strategies and curriculum that ensure safe and effective utilization of new technology. The development of these resources takes a long-term commitment and rapid therapy integration is not always possible, largely based on the complexity of the technology. With regards to challenges around patient access to technology and reimbursement, this is a complex subject that will ultimately require intricate cross-collaboration between multiple stakeholders including patient advocacy groups, legislators, and device manufacturers in order to “move the needle.”
Cynthia: There are many on-going initiatives aimed at these barriers- this article and platform being one of them! My view is education, education, education. This may be seen as too simplistic, but I believe that as our society becomes more knowledgeable it improves the care for all. If you look at society over time, we have made amazing strides both socially and medically. I would like us to become more innovative with education and information sharing. We get information from so many different outlets these days and depending on our background, comfort level and stage of life we listen more intently to diverse sources. A great outcome would be information being shared by medical outlets, religious groups, social advocacy groups and mainstream media. People also have different learning styles and preferences, so ensuring that everyone is able to access the medical information and education that they need in the way they best understand it would enable greater understanding and decision making. If all these avenues are dovetailing into one another but presenting the information with a slightly different vantage point, you can reach more Americans authentically and change patterns of health for the better.
Alex: Manufacturers, providers, and policymakers can work to overcome these barriers. Manufacturers need to carefully consider the economic realities of providing care for ESRD patients during device development, and aim to keep per-encounter costs down through intentional design choices. Policymakers can further support this by incentivizing design innovation (e.g. new devices and technologies) in addition to the ongoing incentives for structural innovation (e.g. shifting dialysis care to home). Providers can be supportive by being open to new technologies, including being active participants in clinical trials and pilot launches of new devices. Limited launches for devices can be critical for generating the necessary evidence to fuel subsequent widespread adoption. Manufacturers need to be mindful of the production pressure for ESRD providers and ensure that devices integrate well into existing workflows, and minimize learning curve and logistical burdens associated with device use.
Eric: Novel devices and approaches that challenge our current state of thinking are direly needed. For example, it is well known that long term outcomes of hemodialysis are not optimal and 5-year and 10-year mortality continue to be high, mainly due to cardiovascular disease (CVD). Quality of life of patients undergoing dialysis are continue to be poor, especially chronic fatigue which can be debilitating. A significant pathological factor is the underlying chronic systemic inflammation that continues to drive not only CVD, but also is an important driver of fatigue. By targeting chronic systemic inflammation, we can hope to improve not only long-term outcomes in chronic dialysis, but also improve their quality of life and make it a bit easier for them to undergo a treatment that is life-saving, yet can be challenging to undergo.
David: Early multidisciplinary collaboration, particularly including CMS and other payors, can optimize evidence development strategies and provide greater certainty around market access. The FDA has created several successful programs (e.g. Q-Sub, ombudsman, BDD) that would provide tremendous value if replicated in the payor environment.3
Q4. Are current regulatory pathways clear? How and when did you decide on your chosen regulatory path? Who was involved in that decision?
TLDR: Experts acknowledge the importance of aligning regulatory and reimbursement policies with the goal of improving patient outcomes. They suggest that regulatory pathways, while clear, require strategic navigation to mitigate the cost of innovation and to align with fast-advancing technology. The decision-making process often involves extensive company-wide collaboration to consider factors such as the novelty of the device and potential strategic advantages. Early engagement with regulatory bodies like the FDA is crucial, as is the use of their mechanisms and guidance documents for clarity in product development. The collective focus remains on partnership and shared accountability between device makers and regulatory entities, ensuring innovation progresses without compromising quality and safety.
John: Regulatory and reimbursement policy need to be aligned with desired outcomes. From a regulatory perspective, that means removing hurdles that increase the cost of innovations. From a reimbursement perspective, policy needs to create the right price signals to discourage the creation of negative cost externalities (e.g. fee for service arrangements that focus dialysis providers only on the costs of a dialysis treatment, while insulating them from the cost burden of poorly dialyzed patients needing expensive hospital admissions) and which reward behaviors that are aligned with achieving better outcomes.
Cynthia: I joined after the device was cleared for Home Hemodialysis but in my current role I deal with regulatory planning and updates. When you are trailblazing with technology that is advancing so quickly, it is hard to envision rules for future discoveries and their potential hazards. I am a firm believer in partnership and shared accountability, both device makers and regulatory entities working together for innovation while keeping an eye on quality and safety policy is my continued focus.
Alex: Defining a regulatory strategy can be a long, and iterative process. Deeply studying similar devices, becoming intimately familiar with FDA guidance documents, and then confirming one's plans via Q-submission discussions with the FDA are critical for creating clarity and reducing risk.
Eric: While the regulatory pathways are clear, it is a choice between a 510(k) or Pre-Market Approval (PMA) pathway, and which one to embark on is the key strategic decision for many companies. SeaStar Medical explored both the 510(k) and PMA pathways early in the development for our selective cytopheretic device (SCD) and due to the unexpected clinical results in AKI studies (i.e., reducing mortality rates by 50% and no patient on dialysis at Day 60 in our pediatric study), quickly resolved which pathway to take (i.e. the PMA). The decision involved nearly every function of the company due to the time, cost and resources it takes to receive a PMA versus a 510(k). In our case, there was no predicate device that did what the SCD does - target highly activated pro-inflammatory immune effector cells such as neutrophils and monocytes and deactivates them towards resolution of hyperinflammation, and this is accomplished without immunosuppression or immuno-depletion. At the time, this was seen as something that might be achieved only by using a pharmaceutical drug or biological approach, not a device.
For those starting out, we would suggest engaging with regulatory experts to clearly understand the differences between the 2 major approaches mentioned above, and determining if those differences can provide your device and company with a strategic advantage that makes financial sense.
David: While there are always potential regulatory challenges, the FDA has done an excellent job providing several mechanisms to provide clarity to companies at early stages of product development. There are also an array of guidance documents published by FDA to provide clarity on FDA's expectations across a variety of topics.
Author’s note: You’ll find the FDA guidance documents in the Resources section below.
Q5. Looking ahead, which emerging trends or potential innovations in kidney care excite you most?
TLDR: There is a strong focus on CKD management platforms that facilitate early diagnosis and improved disease management, advocating a home-first approach for ESKD patients. Diagnostic advancements, such as Point of Care and Remote Patient Monitoring solutions, are enhancing patient telemetry. In terms of devices, the development of next-generation dialysis systems is making home treatment more accessible. They also point to the potential of therapeutic breakthroughs, particularly drugs that slow CKD progression and its comorbidities. Further, the potential of patient empowerment through education and technology to inform and enable better self-care is a key trend. Last but not least, innovations targeting chronic systemic inflammation are poised to address the underlying causes of complications in kidney diseases.
John: This is an exciting time for kidney health innovation. One the service side, CKD management platforms are enabling systematic early diagnosis and better disease management to reduce CKD progression and severity, while embracing a home-first approach for ESKD patients. In the diagnostics realm, an array of Point of Care and Remote Patient Monitoring solutions are providing better telemetry on how kidney patients are doing. In the devices space, we're seeing a proliferation next generation dialysis systems that are reducing barriers to treating patients at home. And in the therapeutics realm, new drugs like SGL2 inhibitors and GLP1 agonists are bending the curve in the progression of CKD and its morbidities such as diabetes. Meanwhile, technologies such as gene editing and regenerative medicine are pointing the way to bioengineered replacement kidneys.
Cynthia: I am most excited to see access to information around dialysis explode. While I am equally excited for home care trends and wearable devices like many others, I remain committed to the perspective that if more patients with kidney disease were educated, informed, given access to options and data, they would feel comfortable finding their voice and advocating for their needs. I am not only referring to Home HD. This could mean using a catheter over a fistula due to many social, physical as well as psychological reasons. I think education in this space will lead to individualized care of these patients and a sense of control and comfort when what they mostly experience is loss and grief. Dialysis is a bridging technique that is not health restorative and for that fact alone extremely taxing for patients and their loved ones. Until we can give everyone a new kidney, finding patients' choices and self-preservation must be central in their dialysis journey.
Alex: Patient empowerment (and improving care distribution in general) is a significant trend that I expect to continue to fuel innovation in kidney care. Devices and technologies that further enable patients and their caregivers to do more for themselves will help reduce overall system costs and ultimately distribute care to earlier and earlier timepoints in the patient journey. Patient empowerment has many ingredients, including rich education, timely access to expert knowledge, physical / technical capabilities, and minimized disruption to daily life. Importantly, all of these ingredients arriving together have the best chance of driving meaningful change; they are more effective in concert than standalone.
Eric: For me, I’m most excited about tackling chronic systemic inflammation in kidney diseases. At SeaStar Medical, we’re working on the selective cytopheretic device (SCD), a novel extracorporeal therapy immunomodulatory device that targets highly activated pro-inflammatory immune effector cells such as neutrophils and monocytes and deactivates them towards resolution of hyperinflammation, and this is accomplished without immunosuppression or immuno-depletion. We’re currently studying its effectiveness in addressing the dysregulated hyperinflammatory response in critical care renal situations such as acute kidney injury. Indeed, we are waiting for the final decision (any day) by the FDA for approval for a humanitarian device exemption (HDE) for pediatric AKI in septic patients. This would make the SCD the 1st and only treatment for AKI and septic patients. A true clinical breakthrough. In addition, we have an ongoing pivotal trial in adult AKI with the SCD that is slated to read out in 2025. The SCD has also been granted Breakthrough Device Designation for adult AKI, as well as cardiorenal syndrome and hepatorenal syndrome. We’re excited about this approach because dysregulated inflammatory processes are significant drivers of disease in other renal conditions as well such as CKD, ESRD and dialysis.
As mentioned above, chronic systemic inflammation has been identified as a key driver of cardiovascular disease and fatigue in ESRD patients undergoing chronic dialysis and so the availability of a device such as the SCD now makes it possible to explore targeting systemic inflammation in a manner that is not immunosuppressive. A pilot study with the SCD has already been conducted in 15 ESRD African American patients which demonstrated an anti-inflammatory effect that was durable for up to 2 weeks with just a single 4-hour treatment.
Q6. Story time! I’d love to hear about any experiences or situations where nutrition played a critical role in improving a patient’s life.
John: One of my most memorable experiences was when the family of a home hemodialysis patient reached out to share how home therapy on our device transformed his health and mental well being, giving them their father back.
Michael: My journey into the dialysis world began during my late teens when I was fortunate enough to work as a hemodialysis technician at a hospital in New Jersey. This was truly a formative experience that set in motion my journey to continuously represent the kidney patient community. One particular experience stands out from those early days: the guidance I received from an extraordinary home hemodialysis (HHD) nurse who had undergone a renal transplant herself after being a hemodialysis patient. She not only imparted invaluable knowledge but also became a cherished mentor and friend. Through her lens, I gained profound insights into the significance of treatment options, the importance of flexible care, and the enhancement of quality of life for individuals with end-stage renal disease (ESRD). This perspective deeply influenced me, shaping my dedication to the cause.
Now, as part of the Mozarc Medical team, our mission resonates deeply with my personal journey. With over 25 years of firsthand experience caring for kidney patients, I am humbled to be part of our organization’s commitment to kidney care.
Cynthia: I remember being a first-year intern and listening to my future mentor, an amazing Nephrologist, educate a family on dialysis before beginning treatment. He spent over an hour reviewing terms in ways I never even thought to explain. He reviewed it was ok to have renal failure- its not like "failing" a test, meaning all is lost. He demystified terms and helped them to begin to process their grief. When he began to discuss dialysis as a "bridging technique" and not a restorative health treatment like chemotherapy or surgery or even your nightly vitamins, I became staunch in my view to help these patients. The path to dialysis and then on to transplant is full of "gray", uncertain turns and no easy answers. He taught me to support patients and families through this process and to empower them to achieve their desired outcomes, not mine or what was written in a book.
Alex:
Story 1: I cannot recall a single conversation about in-clinic ESRD care that hasn't included discussion about skill variability across staff members. Patients on hemodialysis quickly learn who is good at cannulating them and who isn't. This can be a source of significant anxiety for patients, as they know that failed cannulation attempts can lead to serious downstream consequences.
Story 2: In any conversation about shifting patients to home hemodialysis, a common refrain from industry veterans has been that fear of self-cannulation is one of the most important barriers to home hemodialysis. An expert in clinical staff education told me that despite doing cannulation on others for decades, the thought of cannulating himself has given him literal nightmares.
Eric: There is a story that fuels me personally. It was the first pediatric patient that was enrolled in our SCD-PED-01 clinical study that received a FDA grant to conduct. The patient was undergoing an elective surgery when she had a drug reaction. That drug reaction lead to 4+ organ failures, with an expected mortality rate over 50%; this was not how the family wanted to spend their weeks right before Christmas. After 7 days on the SCD, the kidney function improved, resulting in complete recovery of kidney function and no requirement for further dialysis treatment. She was discharged from hospital alive with functional recovery of all organs and was able to return to school in January. Each member of SeaStar Medical has a patient story that has touched their lives and passion to do what we do each and every day.
David: Terry Litchfield has been an inspiration to me, my team, and many in the kidney community. She spent many years caring for her husband Bill throughout his time with ESRD and has impressively converted their lived experiences to actionable insights for the innovation and care delivery communities to improve the lives of future patients.
Q7. What else should readers know? What questions would you want your fellow device builders and peers to answer?
As we navigate the complexities of kidney care innovation, I like to ask our experts to share any questions or resources that might spark further discussion. It’s an open mic format, so please feel free to leave a comment if any of these resonate with you. Consider this your invitation to engage in the discussion!
Cynthia asks us to consider:
Education: How can we ensure patients understand all of their options for dialysis (including opting off)?
Advocacy: How can we ensure patients are able to access the type of dialysis they want and freely advocate for themselves?
Equity: How can we help Nephrologists offer all modalities fairly and equitably?
Alex encourages patient involvement:
Ask patients: How do people think about their treatment experiences?
Pain points: What are the top problems patients need help with right now?
Resources
I asked the experts to share their favorite resources and recommendations where readers can learn more about these topics. Here are their must-read recommendations for founders, builders, and key stakeholders:
![Signals From [Space]](https://substackcdn.com/image/fetch/w_80,h_80,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F55686857-6b99-45a6-ac0f-09c9f023f2a0_500x500.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/e_trim:10:white/e_trim:10:transparent/h_72,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F4d588ac1-7fac-4bd4-829d-fc7b4e8f1326_1512x288.png)

![Signals From [Space]](https://substackcdn.com/image/fetch/w_36,h_36,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F55686857-6b99-45a6-ac0f-09c9f023f2a0_500x500.png)