Elliott Levy on career paths in nephrology, the puzzling nature of kidney diseases, and the elegance of clinical research
"We’re now able to target specific pathways in kidney disease that weren’t even identifiable a decade ago."
If you’re a regular Signals reader, you know there’s one area I’ve been more cautious about when it comes to writing about kidney innovation: that is, biopharma & life sciences. Well, I have some good news.
Today I’m excited to share my first in a series of collaborations with Dr. Janis Naeve, a scientist-turned-venture capitalist who has backed companies across the lifecycle in life sciences and kidney disease. Having served in leadership roles at Amgen Ventures, Cota Capital, and KidneyX, and on the Board at companies like Algen Bio, Mission Bio, Excision Bio, Probius, and CellFE, we’re lucky to have Janis as our guide into the fascinating and groundbreaking world of biopharma, biotech, and life sciences.
For our first piece, we wanted to share a story. Our goal was to highlight nontraditional career paths in nephrology; instead, we rediscovered why people fall in love with kidney care in the first place. I’m grateful Janis said ‘yes’ to co-writing this series; thankful Dr. Levy shared his journey; and excited to pass along what we learned from a career spanning decades, disciplines, disease areas, and several drug development firsts.
As always, thank you for being here and for being part of this conversation. If this interview, topic, or format resonates with you, we’d love to hear from you in the comments below. Keep exploring.
— Tim
Meet Elliott Levy
Dr. Elliott Levy has nearly 25 years of leadership experience in the pharmaceutical industry, where he made significant contributions to the development of over 20 approved medicines across 7 therapeutic areas during his tenure at Amgen and Bristol Myers Squibb. Elliott also contributed to the development of the first modern immunotherapy for cancer, and the first new cornerstone transplant therapy in 25 years.
Since 2022, Dr. Levy has served as a Venture Partner at 5AM Ventures, a leading investment firm focused on building next-generation life sciences companies. But before leading R&D strategy in the life sciences industry, Dr. Levy was trained and certified in internal medicine and nephrology. His career arc from clinical medicine and research into the pharmaceutical industry is what we wanted to explore today.
What you’ll learn today
You've had a fascinating career, from your early days in academia to your work in the pharmaceutical industry. Could you start by sharing your career trajectory and what led you into nephrology?
EL: Sure! I trained in internal medicine and nephrology at Yale and later joined the renal division at Brigham and Women’s Hospital, Harvard. My career initially focused on academic medicine, balancing clinical care, teaching, and research. In 1997, I transitioned into the pharmaceutical industry, driven by a desire to focus on clinical research at a larger scale. I spent 17 years at Bristol-Myers Squibb, mainly outside of nephrology, since there wasn’t much R&D in kidney disease at the time. Eventually, I joined Amgen and continued working on clinical development across various therapeutic areas.
What inspired you to specialize in nephrology during your medical training?
EL: It was the elegance of nephrology that drew me in. The field allows you to explain complex metabolic disturbances through a detailed understanding of kidney function. For decades, nephrology was where physiology met clinical medicine, especially for patients with disorders beyond end-stage renal disease. The scientific rigor and the intellectual challenge appealed to me greatly. Nephrology attracted people who loved to solve puzzles, and the elegance of understanding and treating kidney diseases was incredibly satisfying.1
You transitioned from academia to the pharmaceutical industry. How did your work change after leaving academia?
EL: The transition was significant. At Bristol-Myers Squibb, I focused on large-scale clinical trials, which was a huge shift from the smaller-scale work I had been doing in academia. I moved from nephrology-specific research to broader projects, including developing antihypertensive drugs. The opportunity to work on large-scale trials without the administrative burden of academia was very appealing. I could focus exclusively on research and clinical development, which was rewarding.
Given your experience, what do you see as the most significant shifts in nephrology research over the years?
EL: There have been several important changes. One of the biggest is the increased awareness and earlier detection of chronic kidney disease (CKD). There’s now a heightened focus on diagnosing CKD earlier and optimizing treatment, even for patients with normal or slightly elevated creatinine levels. This shift is due in part to the adoption of more accurate diagnostic formulas and a greater understanding of kidney disease progression. Another significant change is the realization that drugs developed for smaller, targeted populations can be profitable, which has encouraged more investment in nephrology research.2

You mentioned the shift in the regulatory landscape. How has that impacted drug development in nephrology?
EL: The regulatory environment has shifted in a way that’s more conducive to developing drugs for chronic kidney disease. For example, it’s now possible to gain approval for a CKD drug based on a surrogate endpoint, like proteinuria, with full approval following based on slowing the decline in glomerular filtration rate (GFR), rather than requiring long-term outcomes data. This has lowered the barrier to bringing new therapies to market, making it more feasible for companies to invest in nephrology. It’s a profound shift that’s opening up new opportunities for innovation in the field.3
With so much innovation happening, how do you see real-world data playing a role in clinical research moving forward?
EL: Real-world data is going to be crucial in optimizing the use of new treatments. We have more treatment options than ever before, including SGLT2 inhibitors and GLP-1 agonists, which have shown renal protective effects. But the question remains: When should these treatments be started? Who are the best candidates for these therapies? Real-world data will help us answer these questions by providing insights from everyday clinical practice, which can guide better decision-making.
There’s also a lot of potential for real-world data to help us understand the long-term outcomes of these treatments. Clinical trials provide valuable information, but they’re limited by time and scale. Real-world data allows us to see how these therapies perform in broader patient populations over longer periods, which will be key to refining our approach to treating CKD and other conditions.

What do you think is the next big breakthrough we can expect in nephrology?
EL: The next big breakthrough will likely come from continued advancements in precision medicine. As we learn more about the genetic and molecular underpinnings of kidney disease, we’ll be able to develop therapies that are tailored to specific patient subgroups. This personalized approach will revolutionize how we treat kidney disease and improve outcomes for patients who currently have limited treatment options. Additionally, I believe we’ll see more cross-disciplinary collaboration between nephrology and other fields, such as cardiology and oncology, leading to even more innovative therapies.
Let’s look to the future. What excites you the most about where nephrology and biotech innovation are headed?
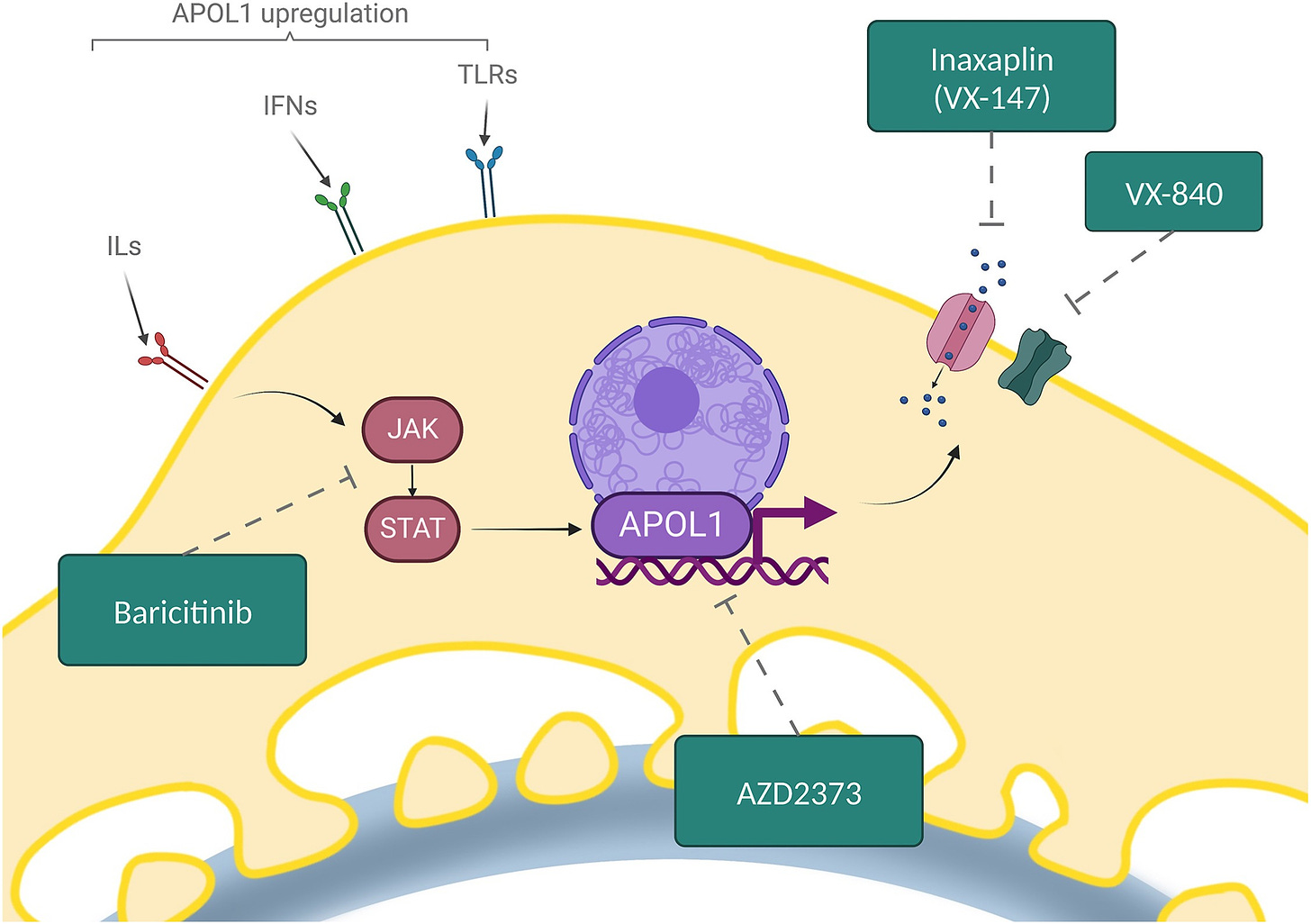
EL: I’m particularly excited about the pace of innovation in the biotech space, especially in nephrology. We’re seeing new therapies being developed that target specific pathways in kidney disease, something that wasn’t possible even a decade ago. The science behind nephrology is evolving rapidly. We’re now able to identify specific targets, like the APOL1 gene, which has been implicated in certain kidney diseases. This understanding opens up possibilities for targeted therapies that can address the underlying causes of kidney disease rather than just managing symptoms.4
What’s also exciting is the growing recognition that smaller patient populations can be profitable. This has encouraged investment in rare diseases and niche areas within nephrology, leading to breakthroughs that would have been considered too risky in the past. The future of nephrology is not just about treating kidney failure but about intervening earlier and potentially reversing the course of the disease with innovative therapies.
Keep Exploring
We put together a few resources based on topics covered in our Q&A, then asked Elliott to include a few of his recommendations as well. Enjoy!
Mind the gap in kidney care: translating what we know into what we do. Historically, it takes an average of 17 years to move new treatments from clinical evidence to daily practice. Given the highly effective treatments now available to prevent or delay kidney disease onset and progression, this is far too long. The time is now to narrow the gap between what we know and what we do. A paper published for the World Kidney Day joint steering committee discusses how systemic barriers must be acknowledged so that sustainable solutions are developed and implemented without further delay.
Advancing Genetic Testing in Kidney Diseases: Report From a National Kidney Foundation Working Group. The National Kidney Foundation (NKF) has released new comprehensive recommendations for the use of genetic testing in diagnosing genetic kidney diseases. About 10% or more of kidney diseases in adults and about 70% of certain chronic kidney diseases in children are expected to be explained by genetic causes.
A Practical Guide to Genetic Testing for Kidney Disorders of Unknown Etiology. Genetic testing is increasingly used in the workup and diagnosis of kidney disease and kidney-related disorders of undetermined cause. This review aims to guide clinicians in formulating pretest conversations with their patients, interpreting genetic variant nomenclature, and considering follow-up investigations.
A comparative study of clinical trial and real-world data in patients with diabetic kidney disease. A growing body of research is focusing on real-world data (RWD) to supplement or replace randomized controlled trials (RCTs). This analysis compared the characteristics of RCT data from 5734 diabetic kidney disease patients with corresponding RWD from electronic health records (EHRs) of 23,523 patients.
Surrogate Endpoint Resources for Drug and Biologic Development. Surrogate endpoints are used in clinical trials submitted to support either traditional or accelerated approval of drugs and biologics. Under Section 507 of the Federal Food, Drug, and Cosmetic Act, as amended by the 21st Century Cures Act, FDA must make public a list of “surrogate endpoints which were the basis of approval or licensure (as applicable) of a drug or biological product.”
Breakthrough Discoveries in Nephrology — ISN Research Working Group (2021)
Buvall L, Menzies RI, Williams J, Woollard KJ, Kumar C, Granqvist AB, Fritsch M, Feliers D, Reznichenko A, Gianni D, Petrovski S, Bendtsen C, Bohlooly-Y M, Haefliger C, Danielson RF and Hansen PBL (2022) Selecting the right therapeutic target for kidney disease. Front. Pharmacol. 13:971065. doi: 10.3389/fphar.2022.971065
Vasquez-Rios, G., De Cos, M., & Campbell, K. N. (2023). Novel Therapies in APOL1-Mediated Kidney Disease: From Molecular Pathways to Therapeutic Options. Kidney International Reports, 8(9), 2226-2234. https://doi.org/10.1016/j.ekir.2023.08.028
![Signals From [Space]](https://substackcdn.com/image/fetch/w_80,h_80,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F55686857-6b99-45a6-ac0f-09c9f023f2a0_500x500.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/e_trim:10:white/e_trim:10:transparent/h_72,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F4d588ac1-7fac-4bd4-829d-fc7b4e8f1326_1512x288.png)

![Signals From [Space]](https://substackcdn.com/image/fetch/w_36,h_36,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F55686857-6b99-45a6-ac0f-09c9f023f2a0_500x500.png)