Signals Brief: Why Lux Capital is Betting Big on CRISPR and the Future of Organ Transplants
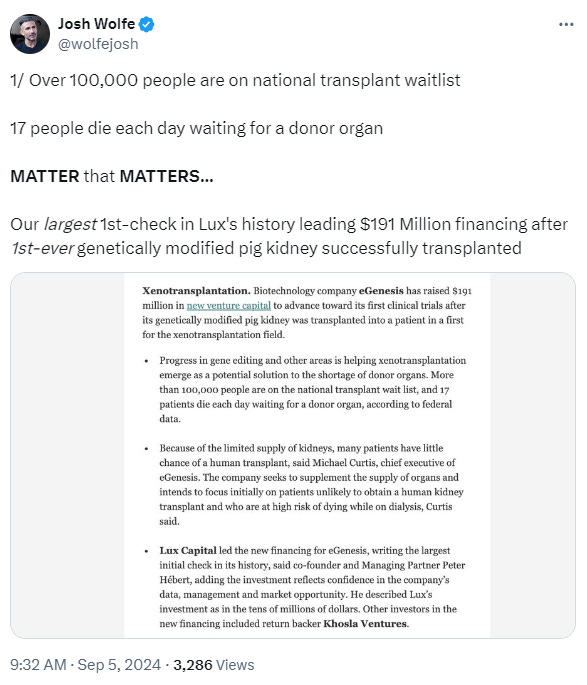
eGenesis just raised $191M from a stellar group of investors betting on a future where 'dialysis becomes obsolete.'
Kidney care is witnessing another overnight success, decades in the making. Recent advances in gene-editing, policy reform, new care models, and drug development are converging, pushing the boundaries of what’s possible for patients over the next decade. This week’s brief is about momentum. With this latest funding, eGenesis aims to move genetically edited pig kidneys into human trials, advance pipeline programs, and scale production of its solution to the organ supply crisis.
Ten years ago, George Church and Luhan Yang shared their groundbreaking findings on inactivating 62 genes in pig embryos. Since then, they co-founded eGenesis to make this process more affordable— that company has raised close to $500 million to turn that vision into reality. In 2015, Church said, 'This is something I’ve been wanting to do for almost a decade.' Here we are, a generation later, and that company is moving mountains, sixty-nine genes at a time.
In this brief, we dive into what this news means for kidney care, its potential for shaping new treatment models, and why this moment feels like a turning point for an industry that’s long overdue for new realities.
What Happened
eGenesis has just raised $191M to push their lead product, a genetically modified pig kidney, into human trials. This funding round marks a big step for the company as it aims to solve the global organ shortage. Earlier this year, eGenesis made history by successfully transplanting its first pig kidney into a human. Now, with nearly $500 million raised to date, the company’s lead product candidate, EGEN-2784, is poised for it’s next big milestone: first in-human trials.
This Series D financing round was led by Lux Capital, a top-tier venture firm that’s backed more than 200 companies creating the future through science and technology, including human health. Joining the round were DaVita, one of the largest kidney care providers in the U.S., and a roster of existing investors that included Fresenius, who led their $100M Series B in 2019.
Brick by brick, the transplant landscape is being rebuilt before our eyes. It feels like each week brings us another chapter in this story — and one step closer to rewriting the narrative of kidney failure.
Let’s jump in.
Why It Matters
There are 100,000 people waiting for an organ in the U.S., and too many don’t live to get the call. The organ shortage for end-stage kidney disease (ESKD) patients has plagued healthcare for decades. Xenotransplantation—using animal organs in humans—has long been a pipe dream, but technical and ethical hurdles have held it back. This latest funding round for eGenesis, however, signals a huge vote of confidence that we may be at a tipping point in science and society.
A generation of Americans has known only one reality: dialysis as the de facto treatment for over 97% of new kidney failure patients.
What if it didn’t have to be that way?
This isn’t just about pig organs; it's about rewriting the future of transplant medicine and kidney care. Investors are betting big on eGenesis, with Lux Capital writing its largest-ever first check, period (cue the goosebumps). That Fresenius, and now DaVita, are backing this effort signals that even incumbents recognize the shifting tides. In 2021, only 12% of eligible dialysis patients made it onto the transplant waitlist, and less than 3% of newly diagnosed kidney failure patients were transplanted. Make no mistake, this technology could change those numbers drastically— but we will need broad involvement from across the kidney care ecosystem to see it through.1
The backing from these major players isn't just about immediate financial gains. It's a long-term bet on the future of medicine and biotech, one that goes beyond kidneys and could pave the way for innovations in other transplantable organs.
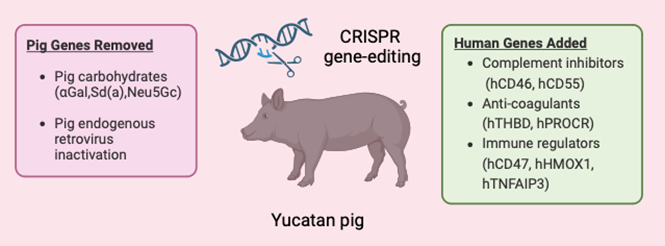
It’s a scientific marvel: 69 genetic edits to the pig genome engineered by eGenesis’ co-founders, George Church and Luhan Yang. With FDA support, human clinical trials are now within reach, marking the dawn of a new era in organ transplantation.23
"Pigs are physiologically close to humans in terms of how their kidneys, hearts and livers work," explains eGenesis CEO Mike Curtis. "And because we can clone pigs, we can do editing to their genomes that makes them compatible. Plus, we've been raising pigs at scale for thousands of years. — Axios
Bigger Picture
Organ scarcity isn’t just a medical issue—it’s a massive economic and social challenge. Not only would xenotransplantation save lives, but it would also reduce the enormous costs of long-term dialysis care. Last week, we learned patients who transition from dialysis to transplant might save Medicare up to $517,000 each, which makes xeno-transplantation a medical and economic marvel. And in case we’re unable to make pig kidneys a scalable solution in the short term, we may soon be able to pay donors for their kidneys. Either way, we need more kidneys with each passing day.
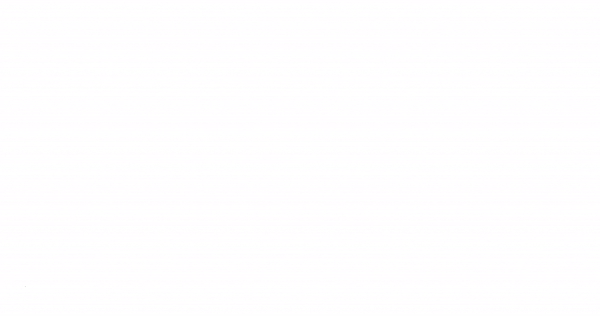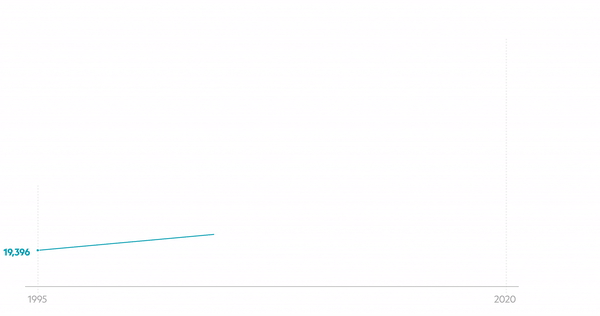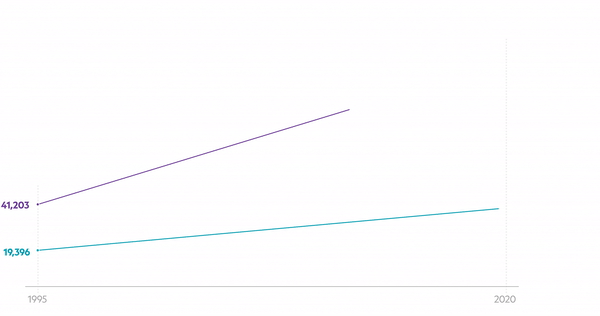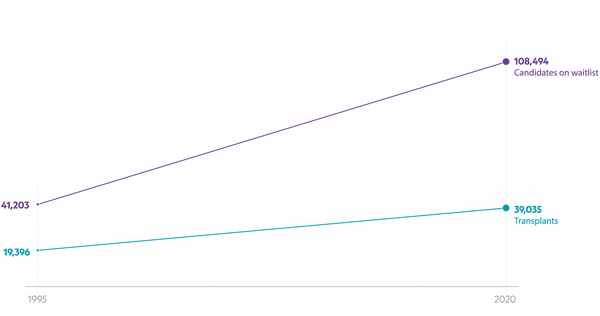
This funding round also underscores the broader trend of convergence between bio-pharma and bio-tech innovation— and those making strategic bets on the future of kidney care. Companies like Fresenius and DaVita play a defining role: together, they control around 80% of the U.S. dialysis clinics and 90% of revenues. A future without dialysis is a bold vision to support. At the same time, biopharma is having a moment in kidney disease, with recent examples like Chinook, Alpine, Calliditas, Borealis, and HI-Bio. The combination of scientific advancement and financial backing provides eGenesis a robust platform to build its pipeline and scale.4
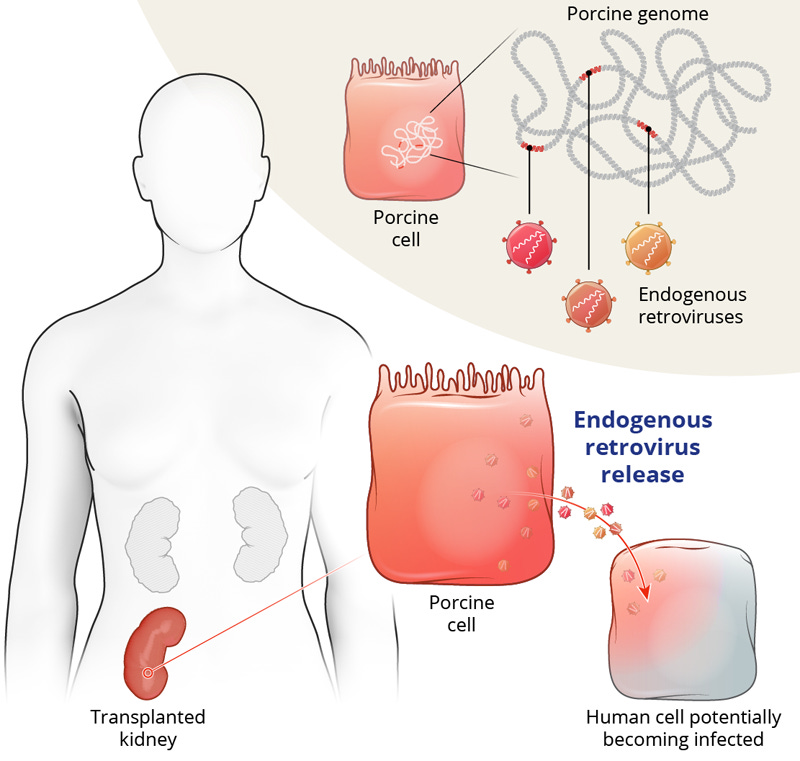
How it works. Using CRISPR-Cas9 technology, scientists made 69 genomic edits to modify pig kidneys for human compatibility. These changes included removing pig genes that trigger immune reactions, adding human genes for better acceptance, and inactivating viral genes present in pig genomes to eliminate infection risks. This breakthrough in gene editing, spearheaded by eGenesis, could reshape organ transplantation for the future.5
The path to clinical trials. The FDA’s decision to approve the compassionate-use of porcine kidney transplantation through expanded access marks an exciting milestone, but there’s still much to explore. Larger, multi-center clinical trials will be essential to assess the safety and long-term effectiveness of xeno-transplantation. As the field progresses, further genetic modifications may be necessary to optimize outcomes for human patients.6
A complex patient journey. While xenotransplantation presents remarkable potential, it's important to acknowledge the complexities. The first-ever human recipient of a genetically engineered pig kidney, Richard “Rick” Slayman, passed away almost two months after the procedure. Although there was no indication his death was the result of the transplant, his doctors hoped it would function for at least two years. These early trials serve as critical learning points that will shape future advancements, further clinical trials, and, with any luck, eventual widespread application.
Beyond kidneys. While eGenesis is currently focused on kidney transplants, their gene-editing technology holds promise beyond just kidneys. The company has programs underway for livers and hearts as well. For pediatric heart patients, xenotransplantation could provide a crucial "bridge" solution while they await a human donor, offering a lifeline to those whose size and availability of organs present significant barriers. As this technology advances, it could reshape transplantation across multiple organ types.7
“Our hope is that dialysis will become obsolete.” — Dr. Leo Riella in NYT
“Xenotransplantation is “one of few” big challenges (along with gene drives and de-extinction, he said) “that really requires the ‘oomph’ of CRISPR.” — George Church in Harvard Gazette
What To Watch
As EGEN-2784 makes its way through clinical and regulatory milestones, here are a few things I’ll be tracking—what will you be watching?
First-in-human trials: eGenesis plans to begin trials for its genetically modified pig kidneys soon. The results of these trials will be critical in determining the viability of xenotransplantation as a solution to the organ shortage crisis.
Regulatory approval pathways: The FDA will be closely watching the outcomes, and a successful first-in-human study could pave the way for accelerated approval pathways, especially considering the high unmet need in kidney transplantation.
Impact on dialysis: If xenotransplantation becomes a viable alternative, how will the dialysis industry adapt? What might this mean for patients, particularly those who have been on waiting lists for years?
Funding for Kidney Biotech: With Lux Capital's entrance and DaVita joining Fresenius as financial backers, we could see an uptick in interest from VCs and healthcare investors in kidney-related biotech. The success of early-stage companies like eGenesis could encourage more funding into technologies with longer commercial timelines. Kidney care
requiresdeserves patient capital.
Discussion
This milestone opens up broader conversation about the role of xenotransplant in medicine and society— how do you think about these topics?
Xeno and ethics: Xenotransplantation presents both promise and controversy. While the potential to save thousands of lives is undeniable, public debates surrounding animal rights, genetic manipulation, and long-term safety persist. How will regulatory bodies and the public respond to these advancements, and what safeguards need to be in place? As eGenesis moves forward, could public perception slow or accelerate the progress of this potentially game-changing solution?
Investor confidence: What does the involvement of major players like Lux Capital and LDOs tell you about kidney care as an investable therapeutic area? What wins or other companies would you point to if you were going to get someone excited about this space?
Kidney care redux: If eGenesis succeeds, what does the future actually look like? What if we doubled or tripled transplants over the next 5 to 10 years? Is that even feasible in the current system? What are the implications for your corner of the kidneyverse?
Thank you for being here. If this conversation resonates, or if you have a story or point of view to share, we’d love to hear from you in the comments below.
Keep exploring,
— Tim
Resources & Data
At the end of each brief I like to share a short list of sites, studies, reports, and other interesting gems I came across while researching the piece. Enjoy!
Breaking Ground in Transplantation: A New Era with Xenotransplantation (NKF). This timeline provides a clear overview of key moments in the journey toward successful xenotransplantation. It highlights breakthroughs, challenges, and ongoing research efforts to address the shortage of viable organs for transplant. If you're following the developments in gene editing and organ transplantation, this piece offers critical context.
First clinical-grade porcine kidney xenotransplant using a human decedent model (AJT). This study highlights both the promising advances and remaining challenges in making xenotransplantation a viable solution. Readers will gain insights into the future of organ transplantation and the barriers researchers are working to overcome.
(2023) USRDS Annual Data Report: Transplantation. The latest ADR contains updated information about the CKD and ESRD populations in the U.S. through the end of 2021. The Transplantation section (ESRD, Chapter 7) examines access to kidney transplantation and outcomes following transplant among patients with ESRD. Analyses span the beginning of the transplant journey (waitlisting) to the end (outcomes after failed kidney transplant).
1H 2024 Venture Healthcare Report (HSBC Innovation Banking). In 2023, venture healthcare faced a challenging IPO market and limited M&A opportunities, grappling with the valuation and investment froth from 2020-1H 2022. However, 1H 2024 saw increased investment across every sector and numerous new investor-led financings. This report has excellent insight into 1H financings across biopharma, diagnostics, devices, and digital health.
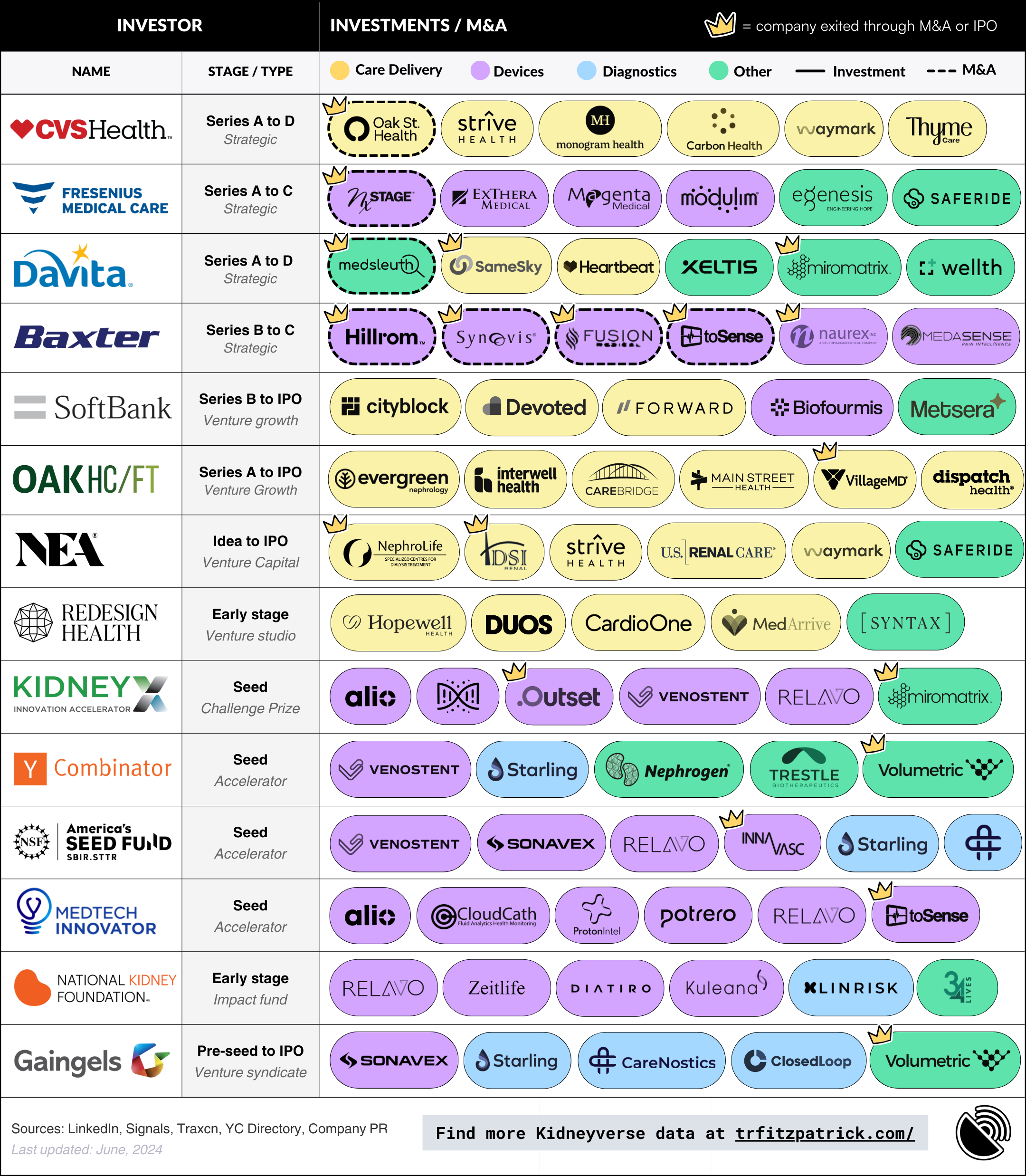
Kidney Capital: Who, When & What Gets Funded In Kidney Care (Signals): A deep dive into the most active investors in the kidney care space, providing context for why this funding round is such a big deal. This is an ongoing series, but the visual below gives you an idea of the types of themes we discuss, from funding gaps to thematic bets.
Want to support Signals?
U.S. Renal Data System, USRDS 2023 Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. https://usrds-adr.niddk.nih.gov/
In a First, Genetically Edited Pig Kidney Is Transplanted Into Human — Harvard Medical School (2024)
Anand, R.P., Layer, J.V., Heja, D. et al. Design and testing of a humanized porcine donor for xenotransplantation. Nature 622, 393–401 (2023). https://doi.org/10.1038/s41586-023-06594-4
How acquisitions affect firm behavior and performance: evidence from the dialysis industry — Harvard Econ (2019)
Questions and Answers about CRISPR — Broad Institute
Porrett PM, Locke JE. A roadmap for human trials of xenotransplantation. J Clin Invest. 2022 Oct 3;132(19):e164484. doi: 10.1172/JCI164484. PMID: 36189798; PMCID: PMC9525115.
![Signals From [Space]](https://substackcdn.com/image/fetch/w_80,h_80,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F55686857-6b99-45a6-ac0f-09c9f023f2a0_500x500.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/e_trim:10:white/e_trim:10:transparent/h_72,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F4d588ac1-7fac-4bd4-829d-fc7b4e8f1326_1512x288.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/w_36,h_36,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F55686857-6b99-45a6-ac0f-09c9f023f2a0_500x500.png)